Cell Reports ( IF 8.8 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.celrep.2020.107840 Pengrong Yan 1 , Hardik J Patel 1 , Sahil Sharma 1 , Adriana Corben 2 , Tai Wang 1 , Palak Panchal 1 , Chenghua Yang 3 , Weilin Sun 1 , Thais L Araujo 1 , Anna Rodina 1 , Suhasini Joshi 1 , Kenneth Robzyk 1 , Srinivasa Gandu 1 , Julie R White 4 , Elisa de Stanchina 5 , Shanu Modi 6 , Yelena Y Janjigian 6 , Elizabeth G Hill 7 , Bei Liu 8 , Hediye Erdjument-Bromage 9 , Thomas A Neubert 9 , Nanette L S Que 10 , Zihai Li 8 , Daniel T Gewirth 10 , Tony Taldone 1 , Gabriela Chiosis 11
|
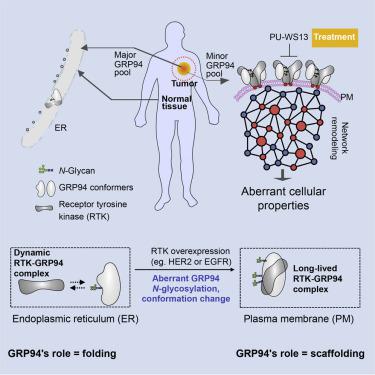
Stresses associated with disease may pathologically remodel the proteome by both increasing interaction strength and altering interaction partners, resulting in proteome-wide connectivity dysfunctions. Chaperones play an important role in these alterations, but how these changes are executed remains largely unknown. Our study unveils a specific N-glycosylation pattern used by a chaperone, Glucose-regulated protein 94 (GRP94), to alter its conformational fitness and stabilize a state most permissive for stable interactions with proteins at the plasma membrane. This “protein assembly mutation’ remodels protein networks and properties of the cell. We show in cells, human specimens, and mouse xenografts that proteome connectivity is restorable by inhibition of the N-glycosylated GRP94 variant. In summary, we provide biochemical evidence for stressor-induced chaperone-mediated protein mis-assemblies and demonstrate how these alterations are actionable in disease.
中文翻译:

分子应激通过伴侣的异常 N-糖基化导致蛋白质连接功能障碍。
与疾病相关的压力可能通过增加相互作用强度和改变相互作用伙伴来病理性地重塑蛋白质组,从而导致蛋白质组范围内的连接功能障碍。伴侣蛋白在这些改变中起着重要作用,但这些改变是如何执行的在很大程度上仍然未知。我们的研究揭示了伴侣蛋白葡萄糖调节蛋白 94 (GRP94) 使用的特定N糖基化模式,以改变其构象适应性并稳定最允许与质膜上蛋白质稳定相互作用的状态。这种“蛋白质组装突变”重塑了蛋白质网络和细胞的特性。我们在细胞、人类标本和小鼠异种移植物中表明,蛋白质组连通性可以通过抑制N-糖基化 GRP94 变体。总之,我们为应激源诱导的分子伴侣介导的蛋白质错误组装提供了生化证据,并证明了这些改变如何在疾病中发挥作用。

























 京公网安备 11010802027423号
京公网安备 11010802027423号