当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
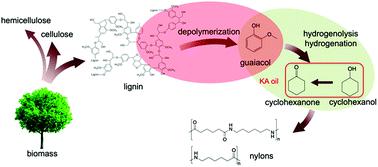
Two-step conversion of Kraft lignin to nylon precursors under mild conditions
Green Chemistry ( IF 9.8 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0gc01220c Hui Zhou 1, 2, 3 , Hsin Wang 1, 2, 3, 4, 5 , Frédéric A. Perras 1, 2, 3 , Pranjali Naik 1, 2, 3, 4, 5 , Marek Pruski 1, 2, 3, 4, 5 , Aaron D. Sadow 1, 2, 3, 4, 5 , Igor I. Slowing 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0gc01220c Hui Zhou 1, 2, 3 , Hsin Wang 1, 2, 3, 4, 5 , Frédéric A. Perras 1, 2, 3 , Pranjali Naik 1, 2, 3, 4, 5 , Marek Pruski 1, 2, 3, 4, 5 , Aaron D. Sadow 1, 2, 3, 4, 5 , Igor I. Slowing 1, 2, 3, 4, 5
Affiliation
|
This study explores the valorization of Kraft lignin by conversion into nylon precursors in a two-step process. First, lignin was depolymerized in dilute alkaline aqueous solution under atmospheric N2 at 200 °C to give guaiacol with high selectivity (>80%) with a total monomer production of 13% based on lignin input. Solution and solid state NMR analyses and reactions of model compounds indicated that depolymerization took place via cleavage of β-O-4 bonds in lignin. In the second step, lignin-derived guaiacol was selectively converted to the nylon precursors cyclohexanol/cyclohexanone (KA oil) using Ru/C catalyst under 1 bar H2 and 150 °C. This two-step process constitutes a low-temperature and low-pressure pathway for producing value-added chemicals from lignin using water as the reaction solvent.
中文翻译:

在温和条件下将牛皮纸木质素分两步转化为尼龙前体
这项研究探索了牛皮纸木质素在两步法中转化为尼龙前体的价值。首先,将木质素在200°C的大气压N 2下于稀碱性水溶液中解聚,从而得到具有高选择性(> 80%)的愈创木酚,基于木质素输入量,总单体产量为13%。溶液和固态NMR分析以及模型化合物的反应表明,解聚反应是通过木质素中的β-O-4键断裂而发生的。在第二步中,使用Ru / C催化剂在1 bar H 2下将木质素衍生的愈创木酚选择性转化为尼龙前体环己醇/环己酮(KA油)和150°C。这两个步骤的过程构成了低温和低压途径,用于使用水作为反应溶剂从木质素生产增值化学品。
更新日期:2020-07-20
中文翻译:

在温和条件下将牛皮纸木质素分两步转化为尼龙前体
这项研究探索了牛皮纸木质素在两步法中转化为尼龙前体的价值。首先,将木质素在200°C的大气压N 2下于稀碱性水溶液中解聚,从而得到具有高选择性(> 80%)的愈创木酚,基于木质素输入量,总单体产量为13%。溶液和固态NMR分析以及模型化合物的反应表明,解聚反应是通过木质素中的β-O-4键断裂而发生的。在第二步中,使用Ru / C催化剂在1 bar H 2下将木质素衍生的愈创木酚选择性转化为尼龙前体环己醇/环己酮(KA油)和150°C。这两个步骤的过程构成了低温和低压途径,用于使用水作为反应溶剂从木质素生产增值化学品。



























 京公网安备 11010802027423号
京公网安备 11010802027423号