当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed asymmetric hydrophosphorylation of alkynes: facile access to P-stereogenic phosphinates
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0sc01049a Zhiping Yang 1, 2 , Xiaodong Gu 2 , Li-Biao Han 3 , Jun Joelle Wang 2
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0sc01049a Zhiping Yang 1, 2 , Xiaodong Gu 2 , Li-Biao Han 3 , Jun Joelle Wang 2
Affiliation
|
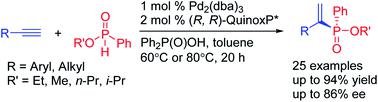
Despite the importance of P-chiral organophosphorus compounds in asymmetric catalysis, transition metal-catalyzed methods for accessing P-chiral phosphine derivatives are still limited. Herein, a catalytic enantioselective method for the synthesis of P-stereogenic alkenylphosphinates is developed through asymmetric hydrophosphorylation of alkynes. This process is demonstrated for a wide range of racemic phosphinates and leads to diverse P-stereogenic alkenylphosphinates directly.
中文翻译:

钯催化的炔烃不对称氢磷酸化:轻松获得 P-立体次膦酸盐
尽管P-手性有机磷化合物在不对称催化中很重要,但过渡金属催化的获取P-手性膦衍生物的方法仍然有限。在此,通过炔烃的不对称氢磷酸化开发了一种催化对映选择性合成P-立体烯基次膦酸盐的方法。该过程已针对多种外消旋次膦酸盐得到证实,并直接产生多种P-立体烯基次膦酸盐。
更新日期:2020-07-22
中文翻译:

钯催化的炔烃不对称氢磷酸化:轻松获得 P-立体次膦酸盐
尽管P-手性有机磷化合物在不对称催化中很重要,但过渡金属催化的获取P-手性膦衍生物的方法仍然有限。在此,通过炔烃的不对称氢磷酸化开发了一种催化对映选择性合成P-立体烯基次膦酸盐的方法。该过程已针对多种外消旋次膦酸盐得到证实,并直接产生多种P-立体烯基次膦酸盐。

























 京公网安备 11010802027423号
京公网安备 11010802027423号