当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multicomponent benzannulation of allylic P-ylides with isocyanates or aldehydes for construction of anilines and biaryls.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0cc03461d Kaki Raveendra Babu 1 , Yang Li 1 , Wenbo Xu 2 , Yuhai Tang 1 , Wenquan Zhang 3 , Silong Xu 1
Chemical Communications ( IF 4.9 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0cc03461d Kaki Raveendra Babu 1 , Yang Li 1 , Wenbo Xu 2 , Yuhai Tang 1 , Wenquan Zhang 3 , Silong Xu 1
Affiliation
|
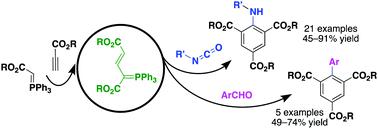
The reactivity of allylic phosphorus ylides generated in situ from alkoxycarbonylmethylenephosphoranes and propiolates is investigated toward isocyanates and aromatic aldehydes, which leads to one-pot multicomponent benzannulations for efficient synthesis of polysubstituted anilines and biaryls, respectively. The mechanism may involve a tandem [2+2] cycloaddition/fragmentation/Wittig/cyclization/elimination/aromatization sequence.
中文翻译:

烯丙基对异戊二烯与异氰酸酯或醛的多组分苯环用于苯胺和联芳基的构建。
研究了由烷氧基羰基亚甲基正膦和丙酸酯在原位生成的烯丙基磷酰化物对异氰酸酯和芳族醛的反应性,这导致了单罐多组分苯环用于分别高效合成多取代苯胺和联芳基。该机制可能涉及串联[2 + 2]环加成/片段化/ Wittig /环化/消除/芳香化序列。
更新日期:2020-08-04
中文翻译:

烯丙基对异戊二烯与异氰酸酯或醛的多组分苯环用于苯胺和联芳基的构建。
研究了由烷氧基羰基亚甲基正膦和丙酸酯在原位生成的烯丙基磷酰化物对异氰酸酯和芳族醛的反应性,这导致了单罐多组分苯环用于分别高效合成多取代苯胺和联芳基。该机制可能涉及串联[2 + 2]环加成/片段化/ Wittig /环化/消除/芳香化序列。


























 京公网安备 11010802027423号
京公网安备 11010802027423号