Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2020-05-31 , DOI: 10.2174/1570180816666191024090857 Nimisha jain 1 , Pradeep Kumar Singour 1
|
Background: According to the World Health Organization, 50 million people worldwide are suffering from epilepsy, making it one of the most common neurological diseases globally. 2,3 disubstituted quinazolinone-4-one derivatives endowed with various pharmacological activity, particularly having anticonvulsant action.
Objectives: The aim of this study was to synthesize 3-Substituted-2,3-Dihydro-2-thioxoquinazolin- 4-(1H)-one derivative and evaluate for anticonvulsant activity and neurotoxicity in order to find an efficient, compound with lesser side effects.
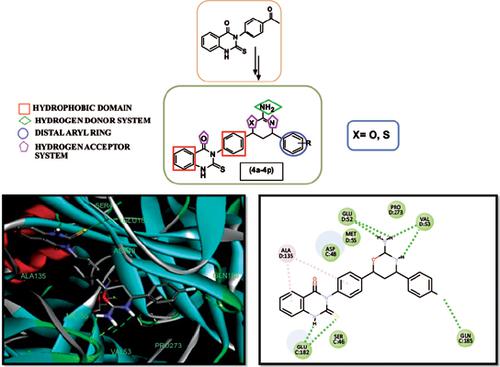
Methods: A novel series of 3-[4-(2-amino-5, 6-dihydro-4(substituted phenyl)-4H-1, 3-oxazin /thiazin-6yl) phenyl]-2, 3-dihyro-2-thioxoquinazolin-4(1H)-one derivatives (4a-4p) were synthesized. The structures of the synthesized compounds were assigned on the basis of spectral data (UV, IR, 1HNMR, 13CNMR and MS) and performed anticonvulsant activity against maximal electroshock test and Subcutaneous Pentylenetetrazole model. Neurotoxicity was assessed using a rotarod apparatus test. The molecular docking study was performed to assess their binding affinities towards Gamma-Aminobutyric Acid type A receptor. A quantitative estimate of drug-likeness was also performed, which calculates the molecular properties and screen the molecules based on drug-likeness rules.
Results: Compounds 4b, 4e, 4j and 4m have shown the highest anticonvulsant activity against tonic seizure with decreased mean duration of tonic hind leg extension of 8.31, 7.35, 8.61 and 8.99 s, respectively in maximal electroshock model and increased onset time clonic convulsion duration of 94.45, 96.65, 93.51 and 91.86 s in Subcutaneous Pentylenetetrazole model. Molecular docking study revealed a better binding affinity with Gamma-Aminobutyric Acid type A receptor.
Conclusion: The compound 4b and 4e emerged out as the pilot molecule with a better anticonvulsant activity without any neurotoxicity. The obtained results showed that compounds 4b and 4e could be useful as a template for future design, optimization, and investigation to produce more active analogs.
中文翻译:

作为抗惊厥药的新型3-取代-2,3-二氢-2-噻吩并喹唑啉-4-(1H)-一衍生物:合成,分子对接和药理筛选
背景:根据世界卫生组织的资料,全世界有5000万人患有癫痫病,使其成为全球最常见的神经系统疾病之一。具有各种药理活性,特别是具有抗惊厥作用的2,3-二取代的喹唑啉酮-4-酮衍生物。
目的:本研究的目的是合成3-取代的2,3-二氢-2-硫代氧杂喹唑啉-4-(1H)-一衍生物,并评估其抗惊厥活性和神经毒性,以便找到一种有效的,具有较少副作用的化合物。效果。
方法:3- [4-(2-氨基-5,6-二氢-4(取代的苯基)-4H-1,3-恶嗪/噻嗪-6基)苯基] -2,3-dihyro-2的新系列合成了-thioxoquinazolin-4(1H)-一衍生物(4a-4p)。根据光谱数据(UV,IR,1HNMR,13CNMR和MS)指定合成化合物的结构,并针对最大电击试验和皮下ent四唑模型表现出抗惊厥活性。使用旋转仪测试评估神经毒性。进行了分子对接研究以评估它们对A型γ-氨基丁酸受体的结合亲和力。还对药物相似性进行了定量估算,该估算可计算分子特性并根据药物相似性规则筛选分子。
结果:化合物4b,4e,4j和4m在最大电击模型中表现出最高的抗惊厥活性,对强直性惊厥的发作具有最大的电击模型,且后肢伸直的平均持续时间分别缩短了8.31、7.35、8.61和8.99 s,而阵发性抽搐持续时间增加了皮下戊四唑模型中94.45、96.65、93.51和91.86 s的时间 分子对接研究显示与A型γ-氨基丁酸受体具有更好的结合亲和力。
结论:化合物4b和4e作为先导分子出现,具有更好的抗惊厥活性,且无神经毒性。获得的结果表明,化合物4b和4e可用作将来设计,优化和研究以生产更多活性类似物的模板。


























 京公网安备 11010802027423号
京公网安备 11010802027423号