当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Surface Reforming via O3 Treatment on the Electrochemical CO2 Reduction Activity of a Ag Cathode
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-06-29 00:00:00 , DOI: 10.1021/acsaem.0c00746 Masaatsu Ishida 1 , Soichi Kikkawa 1, 2 , Kazutaka Hori 1 , Kentaro Teramura 1, 2 , Hiroyuki Asakura 1, 2 , Saburo Hosokawa 1, 2 , Tsunehiro Tanaka 1, 2
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-06-29 00:00:00 , DOI: 10.1021/acsaem.0c00746 Masaatsu Ishida 1 , Soichi Kikkawa 1, 2 , Kazutaka Hori 1 , Kentaro Teramura 1, 2 , Hiroyuki Asakura 1, 2 , Saburo Hosokawa 1, 2 , Tsunehiro Tanaka 1, 2
Affiliation
|
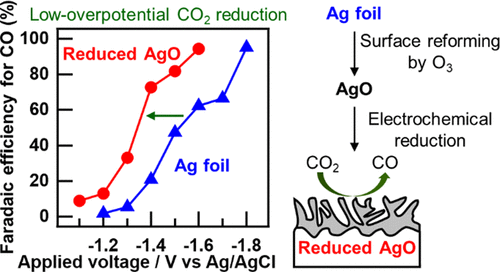
We investigated the effect of O3 oxidation treatment on the electrochemical CO2 reduction activity of a Ag cathode. The surface structure of the Ag electrode was altered using a AgO precursor, which allowed for effective and selective CO2 reduction with high current density. The total current density and faradaic efficiency to CO evolution of the Ag electrode treated using O3 (rO3_Ag electrode) were 4.0 mA cm–2 and 96%, respectively, at an applied voltage of −1.6 V vs Ag/AgCl, whereas those of an untreated Ag foil electrode were 1.3 mA cm–2 and 62%, respectively. Despite enlargement of the electrochemical surface area of the active Ag species by O3 treatment, the turnover frequency of CO evolution using the rO3_Ag electrode was twofold greater than that obtained using the untreated Ag foil electrode. Grazing-incidence X-ray diffraction revealed that the Ag particles grew anisotropically along the [111] direction on the electrode surface due to the reduction of AgO species, which were formed during O3 treatment; this result indicated that the obtained Ag surface was favorable for the electrochemical reduction of CO2 while suppressing H2 production. This simple surface reforming technique enhanced the activity for the electrochemical reduction of CO2 to CO and could be applied in the design of future electrodes for recycling CO2 into feedstocks.
中文翻译:

O 3处理表面重整对Ag阴极电化学CO 2还原活性的影响
我们研究了O 3氧化处理对Ag阴极电化学CO 2还原活性的影响。使用AgO前驱体可以改变Ag电极的表面结构,从而可以在高电流密度下有效,选择性地还原CO 2。在-1.6 V施加电压对Ag / AgCl的条件下,使用O 3(rO 3 _Ag电极)处理的Ag电极的总电流密度和对CO演化的法拉第效率分别为4.0 mA cm –2和96%。未经处理的银箔电极的电导率分别为1.3 mA cm –2和62%。尽管O增加了活性Ag物质的电化学表面积在图3的处理中,使用rO 3 _Ag电极的CO释放的周转频率比使用未处理的Ag箔电极获得的CO的周转频率大两倍。掠入射X射线衍射表明,由于在O 3处理过程中形成的AgO种类减少,Ag颗粒在电极表面沿[111]方向各向异性生长。该结果表明,获得的Ag表面在抑制H 2产生的同时有利于CO 2的电化学还原。这种简单的表面重整技术增强了将CO 2电化学还原为CO的活性,可用于未来回收CO的电极的设计中2成原料。
更新日期:2020-06-29
中文翻译:

O 3处理表面重整对Ag阴极电化学CO 2还原活性的影响
我们研究了O 3氧化处理对Ag阴极电化学CO 2还原活性的影响。使用AgO前驱体可以改变Ag电极的表面结构,从而可以在高电流密度下有效,选择性地还原CO 2。在-1.6 V施加电压对Ag / AgCl的条件下,使用O 3(rO 3 _Ag电极)处理的Ag电极的总电流密度和对CO演化的法拉第效率分别为4.0 mA cm –2和96%。未经处理的银箔电极的电导率分别为1.3 mA cm –2和62%。尽管O增加了活性Ag物质的电化学表面积在图3的处理中,使用rO 3 _Ag电极的CO释放的周转频率比使用未处理的Ag箔电极获得的CO的周转频率大两倍。掠入射X射线衍射表明,由于在O 3处理过程中形成的AgO种类减少,Ag颗粒在电极表面沿[111]方向各向异性生长。该结果表明,获得的Ag表面在抑制H 2产生的同时有利于CO 2的电化学还原。这种简单的表面重整技术增强了将CO 2电化学还原为CO的活性,可用于未来回收CO的电极的设计中2成原料。


























 京公网安备 11010802027423号
京公网安备 11010802027423号