Science of the Total Environment ( IF 9.8 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.scitotenv.2020.140587 Shengnan Chen 1 , Jing Deng 1 , Cheng Ye 1 , Chengcheng Xu 1 , Lingyi Huai 1 , Jun Li 2 , Xueyan Li 3
|
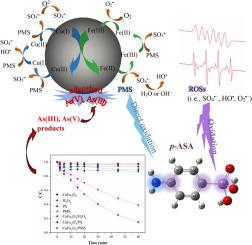
para-arsanilic acid (p-ASA), as a major phenylarsonic feed additive, was used annually in many countries. Once it enters the water environment, p-ASA would be transformed into hypertoxic inorganic arsenic species, causing severe arsenic pollution. In this study, magnetic copper ferrite (CuFe2O4) was applied to activate peroxymonosulfate (PMS) for p-ASA removal and synchronous control of the released inorganic arsenic species. Results showed that CuFe2O4/PMS system presented favorable oxidation ability and close to 85% of 10 mg/L p-ASA was eliminated under the condition of simultaneous dosing 0.2 g/L CuFe2O4 and 1 mM PMS. The rapid decomposition of p-ASA resulted from homogeneous PMS oxidation and the attack of reactive oxygen species (i.e., SO4−, HO
and O2
−), which was involved the heterogeneous PMS activation through the cycles between Fe(II)/Fe(III) and Cu(II)/Cu(I). Meanwhile, the released inorganic arsenic species during p-ASA degradation were found to be controllable via the adsorption on CuFe2O4 surface and metal hydroxyl groups played the crucial role. CuFe2O4/PMS system exhibited the stable and efficient performance within the broad range of pH 3.0–11.0. The existence of common anions (Cl−, NO3−, HCO3−, SO42−) and humic acid presented the slight inhibition for p-ASA degradation. The reduction of initial p-ASA concentration favored the p-ASA removal. Besides, the catalyst retained a favorable reactivity and stability even after four successive cycles and almost no metal leaching was observed. The rational degradation pathway was mainly involved in the cleavage of As
C bond, oxidation of amino group, substitution and oxidation of hydroxyl group. The transformation of arsenic species could be divided into the release of inorganic arsenic species, the oxidation of As(III) into As(V) and the adsorption of As(V) by CuFe2O4.
中文翻译:

通过CuFe2O4活化的过一硫酸盐工艺同时去除对亚砷酸和释放的无机砷物质。
p ARA-阿散酸(p -ASA),作为主要的苯胂饲料添加剂,是每年在许多国家使用。一旦进入水环境,p -ASA将转化为剧毒的无机砷物种,从而导致严重的砷污染。在这项研究中,磁性铜铁氧体(CuFe 2 O 4)用于活化过氧单硫酸盐(PMS)以去除p -ASA和同步控制释放的无机砷物质。结果表明,在同时投加0.2 g / L CuFe 2的条件下,CuFe 2 O 4 / PMS系统具有良好的氧化能力,在10 mg / L p -ASA中几乎消除了85%。O 4和1 mM PMS。迅速分解p -ASA是由于均匀的PMS氧化和反应性氧物质的攻击(即,SO 4 -,HO
和O 2
- ),这是所涉及的异质PMS激活通过周期之间的Fe(II)/ Fe的(III)和Cu(II)/ Cu(I)。同时,发现p -ASA降解过程中释放的无机砷物质可通过在CuFe 2 O 4表面上的吸附来控制,而金属羟基起着至关重要的作用。铜铁2 O 4/ PMS系统在pH 3.0-11.0的宽范围内均表现出稳定而高效的性能。常见阴离子的存在(CL -,NO 3 -,HCO 3 -,SO 4 2-)和腐殖酸呈现为轻微抑制p -ASA降解。初始p -ASA浓度的降低有利于p -ASA的去除。此外,该催化剂甚至在连续四个循环后仍保持良好的反应性和稳定性,并且几乎没有观察到金属浸出。合理的降解途径主要与As的裂解有关
C键,氨基的氧化,羟基的取代和氧化。砷的转化可分为无机砷的释放,As(III)氧化为As(V)以及CuFe 2 O 4吸附As(V)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号