Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-06-27 , DOI: 10.1016/j.cplett.2020.137744 Kyle A. Mason , Adam C. Pearcy , Ka Un Lao , Zachary A. Christensen , M. Samy El-Shall
|
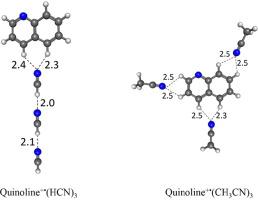
Non-covalent interactions of HCN and CH3CN with the quinoline radical cation (C9H7N+•) through carbon-based ionic hydrogen bonds in the gas phase result in binding energies of 8.1 and 11.5 kcal/mol, respectively. The quinoline cation can be externally solvated with HCN molecules which tend to favor the formation of hydrogen bonding chains rather than multiple direct attachments to the quinoline cation. The solvated structures allow the exposure of the quinoline ion on the surface of cold grains of meteorites and comets to reactive space molecules which could result in the formation of nitrogen-containing complex organic ions in space.
中文翻译:

氰化氢和乙腈通过离子氢键与喹啉自由基阳离子的非共价相互作用
HCN和CH 3 CN通过气相中基于碳的离子氢键与喹啉自由基阳离子(C 9 H 7 N +•)的非共价相互作用导致结合能分别为8.1和11.5 kcal / mol。喹啉阳离子可以被倾向于有利于氢键链形成的HCN分子外部溶剂化,而不是与喹啉阳离子的多个直接连接。溶剂化的结构使喹啉离子暴露在陨石和彗星的冷颗粒表面上的反应性空间分子上,这可能导致空间中含氮复合有机离子的形成。

























 京公网安备 11010802027423号
京公网安备 11010802027423号