当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Boron enhances oxygen evolution reaction activity over Ni foam-supported iron boride nanowires
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-26 , DOI: 10.1039/c9ta14256h Qinghua Liu 1, 2, 3, 4, 5 , Hui Zhao 1, 2, 3, 4 , Meng Jiang 1, 2, 3, 4 , Qing Kang 1, 2, 3, 4 , Wei Zhou 4, 6, 7, 8, 9 , Pengcheng Wang 1, 2, 3, 4 , Feimeng Zhou 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-26 , DOI: 10.1039/c9ta14256h Qinghua Liu 1, 2, 3, 4, 5 , Hui Zhao 1, 2, 3, 4 , Meng Jiang 1, 2, 3, 4 , Qing Kang 1, 2, 3, 4 , Wei Zhou 4, 6, 7, 8, 9 , Pengcheng Wang 1, 2, 3, 4 , Feimeng Zhou 1, 2, 3, 4
Affiliation
|
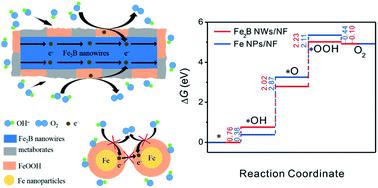
Transition metal borides are one of the most promising electrocatalysts for oxygen evolution reaction (OER), but the precise role of boron is still not understood. Herein, we demonstrate experimentally and computationally that boron facilitates OER by modulating the interaction energies of the reaction intermediates. Through a simple chemical reduction in an externally applied magnetic field, one-dimensional (1D) Fe2B nanowires (NWs) can be directly deposited onto a three-dimensional (3D) nickel foam (NF). OER in alkaline solution converts interconnected Fe2B NWs to a thicker and larger network of metaborate- and FeOOH-covered Fe2B NWs. The Fe2B NWs/NF-based catalyst lowers the overpotential to 276 mV at 10 mA cm−2 (normalized to the electrochemical surface area). This intrinsic catalytic activity ranks top among the transition-metal-based compounds. This catalyst is highly stable, showing only 6 mV change in overpotential over continuous testing for 11 h. Such high efficiency is mainly attributed to the catalyst's unique electronic structure, accelerated charge transport, and hydrophilic surface. The remarkable stability stems from the increase in corrosion resistance by the metaborate species in the catalyst.
中文翻译:

硼增强了镍泡沫支撑的硼化铁纳米线的放氧反应活性
过渡金属硼化物是最有前途的氧释放反应(OER)的电催化剂之一,但硼的确切作用仍不清楚。本文中,我们通过实验和计算证明了硼通过调节反应中间体的相互作用能来促进OER。通过外部施加磁场中的简单化学还原,一维(1D)Fe 2 B纳米线(NWs)可以直接沉积到三维(3D)镍泡沫(NF)上。碱性溶液中的OER会将相互连接的Fe 2 B NW转化为更厚,更大的偏硼酸盐和FeOOH覆盖的Fe 2 B NW网络。Fe 2 B NWs / NF基催化剂在10 mA cm -2时将过电势降至276 mV(归一化为电化学表面积)。这种固有的催化活性在过渡金属基化合物中名列前茅。该催化剂是高度稳定的,在连续测试11小时后,仅显示出6 mV的超电势变化。如此高的效率主要归因于催化剂的独特电子结构,加速的电荷传输和亲水性表面。显着的稳定性源于催化剂中偏硼酸盐物质增加的耐腐蚀性。
更新日期:2020-07-14
中文翻译:

硼增强了镍泡沫支撑的硼化铁纳米线的放氧反应活性
过渡金属硼化物是最有前途的氧释放反应(OER)的电催化剂之一,但硼的确切作用仍不清楚。本文中,我们通过实验和计算证明了硼通过调节反应中间体的相互作用能来促进OER。通过外部施加磁场中的简单化学还原,一维(1D)Fe 2 B纳米线(NWs)可以直接沉积到三维(3D)镍泡沫(NF)上。碱性溶液中的OER会将相互连接的Fe 2 B NW转化为更厚,更大的偏硼酸盐和FeOOH覆盖的Fe 2 B NW网络。Fe 2 B NWs / NF基催化剂在10 mA cm -2时将过电势降至276 mV(归一化为电化学表面积)。这种固有的催化活性在过渡金属基化合物中名列前茅。该催化剂是高度稳定的,在连续测试11小时后,仅显示出6 mV的超电势变化。如此高的效率主要归因于催化剂的独特电子结构,加速的电荷传输和亲水性表面。显着的稳定性源于催化剂中偏硼酸盐物质增加的耐腐蚀性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号