当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Selectivity Patterns in Phosphine-Catalyzed Annulations of Azomethine Imines and Allenoates.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.joc.0c01272 Sebastián Gallardo-Fuentes 1 , Rodrigo Ormazábal-Toledo 1, 2 , Israel Fernández 3
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.joc.0c01272 Sebastián Gallardo-Fuentes 1 , Rodrigo Ormazábal-Toledo 1, 2 , Israel Fernández 3
Affiliation
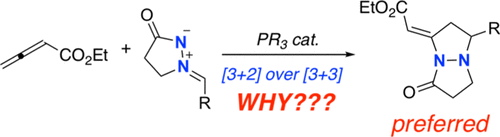
|
The mechanism and selectivity of phosphine-catalyzed [3 + 2] and [3 + 3] annulations of azomethine imines and allenoates have been computationally studied. Exploration of the potential energy surface reveals that the cyclization step is a key step controlling the selectivity of the process. This contrasts with previous studies on related transformations where the initial nucleophilic addition involving the activated allenoate was found to exclusively control the regioselectivity of the transformation. Among the possible reaction pathways, the energetically low-lying reaction channel involves an intramolecular Michael addition leading to the experimentally observed [3 + 2] product. The factors controlling the observed regioselectivity have been quantitatively rationalized by means of state-of-the-art computational methods, namely, the activation strain model of reactivity in combination with the energy decomposition analysis.
中文翻译:

解开膦催化的偶氮亚胺和脲基甲酸酯的环空中的选择性模式。
已通过计算研究了膦催化的[3 + 2]和[3 + 3]环甲亚胺亚胺和脲基甲酸酯环的机理和选择性。对势能面的探索表明,环化步骤是控制过程选择性的关键步骤。这与相关转化的先前研究相反,在相关转化中,涉及活化的烯尿酸酯的最初亲核加成被发现专门控制转化的区域选择性。在可能的反应途径中,能量较低的反应通道涉及分子内迈克尔加成,导致实验观察到的[3 + 2]产物。已经通过最先进的计算方法对控制观察到的区域选择性的因素进行了定量合理化,即
更新日期:2020-07-17
中文翻译:

解开膦催化的偶氮亚胺和脲基甲酸酯的环空中的选择性模式。
已通过计算研究了膦催化的[3 + 2]和[3 + 3]环甲亚胺亚胺和脲基甲酸酯环的机理和选择性。对势能面的探索表明,环化步骤是控制过程选择性的关键步骤。这与相关转化的先前研究相反,在相关转化中,涉及活化的烯尿酸酯的最初亲核加成被发现专门控制转化的区域选择性。在可能的反应途径中,能量较低的反应通道涉及分子内迈克尔加成,导致实验观察到的[3 + 2]产物。已经通过最先进的计算方法对控制观察到的区域选择性的因素进行了定量合理化,即



























 京公网安备 11010802027423号
京公网安备 11010802027423号