当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Large-cavity coronoids with different inner and outer edge structures
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-26 , DOI: 10.1021/jacs.0c05268 Marco Di Giovannantonio 1 , Xuelin Yao 2 , Kristjan Eimre 1 , José I Urgel 1 , Pascal Ruffieux 1 , Carlo A Pignedoli 1 , Klaus Müllen 2, 3 , Roman Fasel 1, 4 , Akimitsu Narita 2, 5
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-26 , DOI: 10.1021/jacs.0c05268 Marco Di Giovannantonio 1 , Xuelin Yao 2 , Kristjan Eimre 1 , José I Urgel 1 , Pascal Ruffieux 1 , Carlo A Pignedoli 1 , Klaus Müllen 2, 3 , Roman Fasel 1, 4 , Akimitsu Narita 2, 5
Affiliation
|
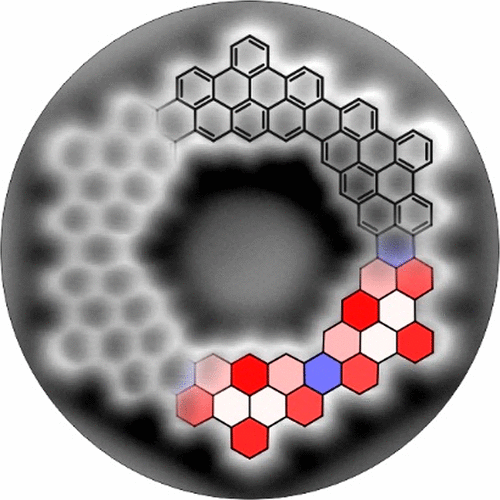
Coronoids, polycyclic aromatic hydrocarbons with geometrically defined cavities, are promising model structures of porous graphene. Here, we report the on-surface synthesis of C168 and C140 coronoids, referred to as [6]- and [5]coronoid, respectively, using 5,9-dibromo-14-phenylbenzo[m]tetraphene as the precursor. These coronoids entail large cavities (>1 nm) with inner zigzag edges, distinct from their outer armchair edges. While [6]coronoid is planar, [5]coronoid is not. Low-temperature scanning tunneling microscopy/spectroscopy and noncontact atomic force microscopy unveil structural and electronic properties in accordance with those obtained from density functional theory calculations. Detailed analysis of ring current effects identifies the rings with the highest aromaticity of these coronoids, whose pattern matches their Clar structure. The pores of the obtained coronoids offer intriguing possibilities of further functionalization toward advanced host–guest applications.
中文翻译:

具有不同内外边缘结构的大腔冠突
Coronoids 是一种具有几何定义空腔的多环芳烃,是多孔石墨烯的有前途的模型结构。在这里,我们报告了 C168 和 C140 类冠酮的表面合成,分别称为 [6]-和 [5] 类冠酮,使用 5,9-二溴-14-苯基苯并 [m] 四苯作为前体。这些冠状突需要大空腔 (>1 nm),内部锯齿形边缘与扶手椅外边缘不同。[6] 冠状面是平面的,而 [5] 冠状面不是。低温扫描隧道显微镜/光谱和非接触式原子力显微镜根据密度泛函理论计算得出的结构和电子特性揭示了结构和电子特性。环电流效应的详细分析确定了这些冠突中芳香性最高的环,其模式与其 Clar 结构相匹配。
更新日期:2020-06-26
中文翻译:

具有不同内外边缘结构的大腔冠突
Coronoids 是一种具有几何定义空腔的多环芳烃,是多孔石墨烯的有前途的模型结构。在这里,我们报告了 C168 和 C140 类冠酮的表面合成,分别称为 [6]-和 [5] 类冠酮,使用 5,9-二溴-14-苯基苯并 [m] 四苯作为前体。这些冠状突需要大空腔 (>1 nm),内部锯齿形边缘与扶手椅外边缘不同。[6] 冠状面是平面的,而 [5] 冠状面不是。低温扫描隧道显微镜/光谱和非接触式原子力显微镜根据密度泛函理论计算得出的结构和电子特性揭示了结构和电子特性。环电流效应的详细分析确定了这些冠突中芳香性最高的环,其模式与其 Clar 结构相匹配。


























 京公网安备 11010802027423号
京公网安备 11010802027423号