当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Matrix Metalloproteinase-Responsive Mesoporous Silica Nanoparticles Cloaked with Cleavable Protein for “Self-Actuating” On-Demand Controlled Drug Delivery for Cancer Therapy
ACS Applied Bio Materials ( IF 4.7 ) Pub Date : 2020-06-26 , DOI: 10.1021/acsabm.0c00497 Kalpesh Vaghasiya 1, 2 , Eupa Ray 1, 2 , Ankur Sharma 1 , Om Prakash Katare 2 , Rahul Kumar Verma 1
ACS Applied Bio Materials ( IF 4.7 ) Pub Date : 2020-06-26 , DOI: 10.1021/acsabm.0c00497 Kalpesh Vaghasiya 1, 2 , Eupa Ray 1, 2 , Ankur Sharma 1 , Om Prakash Katare 2 , Rahul Kumar Verma 1
Affiliation
|
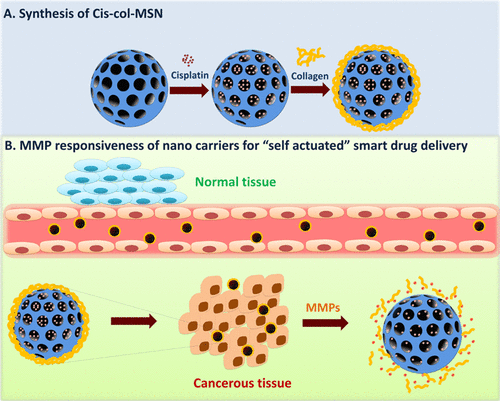
The tumour site-specific stimulus responsiveness of smart drug delivery systems gives a unique system for effective therapeutic delivery with reduced toxic effects of conventional chemotherapeutic drugs. In this work, matrix metalloproteinase-2 (MMP-2)-responsive mesoporous silica nanoparticles (MSNs) were synthesized and assessed for “self-actuating” on-demand controlled drug delivery for cancer therapy. MMPs are members of protease enzymes that are generally overexpressed in cancerous tissues in all stages of cancer. MSNs have attracted significant consideration as a potential delivery system because of their robust and versatile physicochemical properties suitable to deliver the therapeutic payload. Cisplatin (Cis) was used as a model drug, which was incorporated into MSNs to evaluate targeting of lung cancer cells and their release kinetics. In this delivery system, collagen was coated on the surface of Cis-loaded MSNs (Cis-MSN) to form a capping layer, resulting in collagen-coated MSNs (Cis-col-MSN). Under normal cell conditions, a collagen-capping coat efficiently forbids the release of Cis molecules from Cis-col-MSN. The tumor microenvironment would lead to augmented drug release because of the uncapping of collagen from MSN pores due to the presence of overexpressed MMP-2 enzyme and the ensuing controlled drug release. MMP-responsive experiments have shown augmented enzyme triggered drug release. The cellular uptake and cytocompatibility studies in A549 adenocarcinomic lung cancer cell lines demonstrated that this nanocarrier could be efficiently endocytosed in 24 h and have shown favorable biocompatibility with the cells. Cytotoxicity results of Cis-col-MSN demonstrated dose-dependent toxicity. The efficacy of the Cis-col-MSN significantly enhanced with the supplementation of MMP-2 enzyme with increasing concentrations in the cell culture milieu. The efficacy of formulation was attributed to significantly enhance reactive oxygen species, cell cycle arrest, and apoptosis. It is expected that Cis-col-MSN promises a pragmatic approach to constructing an “on-demand” smart drug delivery system to deliver a therapeutic payload at the tumor site only.
中文翻译:

基质金属蛋白酶响应性介孔二氧化硅纳米粒子覆盖有可切割蛋白,用于癌症治疗的“自驱动”按需控制药物递送
智能药物递送系统的肿瘤部位特异性刺激反应性为有效治疗递送提供了独特的系统,同时降低了常规化疗药物的毒性作用。在这项工作中,合成了基质金属蛋白酶-2 (MMP-2) 响应性介孔二氧化硅纳米粒子 (MSN),并评估了用于癌症治疗的“自驱动”按需控制药物递送。MMP 是蛋白酶的成员,通常在癌症的各个阶段在癌组织中过度表达。MSNs 作为一种潜在的递送系统已经引起了人们的广泛关注,因为它们具有强大且通用的物理化学特性,适合递送治疗有效载荷。顺铂 (Cis) 被用作模型药物,将其纳入 MSN 以评估肺癌细胞的靶向性及其释放动力学。在该递送系统中,胶原蛋白被涂覆在载有顺式的 MSN (Cis-MSN) 的表面上以形成覆盖层,从而产生胶原涂层的 MSN (Cis-col-MSN)。在正常细胞条件下,胶原蛋白覆盖层有效地阻止顺式分子从顺-科尔-MSN 中释放。由于过度表达的 MMP-2 酶的存在和随后的受控药物释放,导致 MSN 孔中的胶原蛋白脱壳,肿瘤微环境将导致药物释放增加。MMP 反应实验表明酶促药物释放增强。在 A549 腺癌肺癌细胞系中的细胞摄取和细胞相容性研究表明,这种纳米载体可以在 24 小时内被有效内吞,并显示出与细胞良好的生物相容性。Cis-col-MSN 的细胞毒性结果显示出剂量依赖性毒性。随着 MMP-2 酶在细胞培养环境中浓度的增加,Cis-col-MSN 的功效显着增强。制剂的功效归因于显着增强活性氧、细胞周期停滞和细胞凋亡。预计 Cis-col-MSN 承诺提供一种实用的方法来构建“按需”智能给药系统,仅在肿瘤部位提供治疗有效载荷。
更新日期:2020-08-17
中文翻译:

基质金属蛋白酶响应性介孔二氧化硅纳米粒子覆盖有可切割蛋白,用于癌症治疗的“自驱动”按需控制药物递送
智能药物递送系统的肿瘤部位特异性刺激反应性为有效治疗递送提供了独特的系统,同时降低了常规化疗药物的毒性作用。在这项工作中,合成了基质金属蛋白酶-2 (MMP-2) 响应性介孔二氧化硅纳米粒子 (MSN),并评估了用于癌症治疗的“自驱动”按需控制药物递送。MMP 是蛋白酶的成员,通常在癌症的各个阶段在癌组织中过度表达。MSNs 作为一种潜在的递送系统已经引起了人们的广泛关注,因为它们具有强大且通用的物理化学特性,适合递送治疗有效载荷。顺铂 (Cis) 被用作模型药物,将其纳入 MSN 以评估肺癌细胞的靶向性及其释放动力学。在该递送系统中,胶原蛋白被涂覆在载有顺式的 MSN (Cis-MSN) 的表面上以形成覆盖层,从而产生胶原涂层的 MSN (Cis-col-MSN)。在正常细胞条件下,胶原蛋白覆盖层有效地阻止顺式分子从顺-科尔-MSN 中释放。由于过度表达的 MMP-2 酶的存在和随后的受控药物释放,导致 MSN 孔中的胶原蛋白脱壳,肿瘤微环境将导致药物释放增加。MMP 反应实验表明酶促药物释放增强。在 A549 腺癌肺癌细胞系中的细胞摄取和细胞相容性研究表明,这种纳米载体可以在 24 小时内被有效内吞,并显示出与细胞良好的生物相容性。Cis-col-MSN 的细胞毒性结果显示出剂量依赖性毒性。随着 MMP-2 酶在细胞培养环境中浓度的增加,Cis-col-MSN 的功效显着增强。制剂的功效归因于显着增强活性氧、细胞周期停滞和细胞凋亡。预计 Cis-col-MSN 承诺提供一种实用的方法来构建“按需”智能给药系统,仅在肿瘤部位提供治疗有效载荷。


























 京公网安备 11010802027423号
京公网安备 11010802027423号