当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Halogen Bond‐Catalyzed Povarov Reactions
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-06-25 , DOI: 10.1002/adsc.202000665 Xuelei Liu 1 , Patrick H. Toy 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-06-25 , DOI: 10.1002/adsc.202000665 Xuelei Liu 1 , Patrick H. Toy 1
Affiliation
|
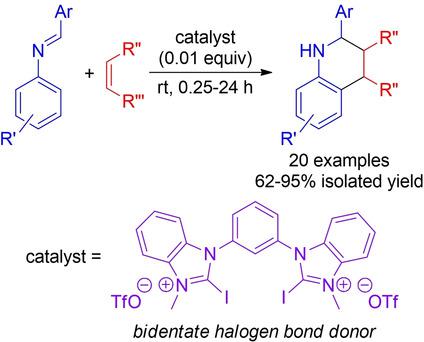
The use of a bidentate halogen bond donor to catalyse Povarov reactions of imines derived from aryl adehydes and anilines is reported. Dienophiles used in these reactions included 2,3‐dihydrofuran, N‐vinyl‐2‐pyrrolidone and N‐Cbz‐protected 2,3‐dihydropyrrole. Very high isolated yields of the desired 1,2,3,4‐tetrahydroquinoline products were obtained with low catalyst loadings (0.01 equivalents) and short reaction times (minutes to hours) at ambient temperature. Notably, the bis(benzimidazolium iodide)‐based catalyst used in these reactions proved to be more efficient than analogous bromine and chlorine functionalized compounds, as well as a related monodentate benzimidizolium iodide. These observations are similar to what has been reported by others regarding halogen bond organocatalysis, and may prove useful in guiding catalyst design as the field moves forward.
中文翻译:

卤素键催化的Povarov反应
据报道,使用双齿卤素键供体来催化衍生自芳基醛和苯胺的亚胺的Povarov反应。在这些反应中使用的亲双烯体包括2,3-二氢呋喃,N-乙烯基-2-吡咯烷酮和NCbz保护的2,3-二氢吡咯。在环境温度下,以较低的催化剂负载量(0.01当量)和较短的反应时间(数分钟至数小时)获得了所需1,2,3,4-四氢喹啉产物的很高的分离产率。值得注意的是,在这些反应中使用的基于双(碘化咪唑鎓)的催化剂被证明比类似的溴和氯官能化化合物以及相关的单齿碘化亚苄基碘更有效。这些观察结果与其他人关于卤素键有机催化的报道相似,随着该领域的发展,可能对指导催化剂设计很有用。
更新日期:2020-08-19
中文翻译:

卤素键催化的Povarov反应
据报道,使用双齿卤素键供体来催化衍生自芳基醛和苯胺的亚胺的Povarov反应。在这些反应中使用的亲双烯体包括2,3-二氢呋喃,N-乙烯基-2-吡咯烷酮和NCbz保护的2,3-二氢吡咯。在环境温度下,以较低的催化剂负载量(0.01当量)和较短的反应时间(数分钟至数小时)获得了所需1,2,3,4-四氢喹啉产物的很高的分离产率。值得注意的是,在这些反应中使用的基于双(碘化咪唑鎓)的催化剂被证明比类似的溴和氯官能化化合物以及相关的单齿碘化亚苄基碘更有效。这些观察结果与其他人关于卤素键有机催化的报道相似,随着该领域的发展,可能对指导催化剂设计很有用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号