当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of Biomimetic Synthesis of Propindilactone G†
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-06-26 , DOI: 10.1002/cjoc.202000293 Yu Wang 1 , Bo Chen 1 , Xubiao He 1 , Jinghan Gui 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-06-26 , DOI: 10.1002/cjoc.202000293 Yu Wang 1 , Bo Chen 1 , Xubiao He 1 , Jinghan Gui 1
Affiliation
|
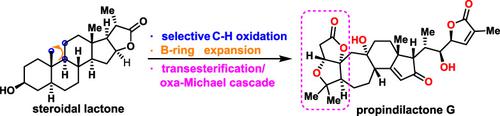
The natural product propindilactone G is a complex Schisandra nortriterpenoid with a unique 5/5/7/6/5 pentacyclic framework. This full paper describes the development of concise biomimetic synthesis of propindilactone G from a known steroid lactone. The key C19‐OH intermediate was synthesized via Breslow and Suárez radical remote C—H functionalizations. Wagner‐Meerwein rearrangement was subsequently utilized for the expansion of the B ring. To invert the configuration of the C10 tertiary alcohol, an intramolecular peroxide cyclization catalyzed by BF3·Et2O was devised. The 5/5 fused lactone system was then assembled in a biomimetic transesterification/oxa‐Michael addition sequence. Our work should provide experimental support for the proposed biosynthetic pathway and facilitate investigation of the biological activities of propindilactone G.
中文翻译:

仿丙内酯G†仿生合成的研究进展
天然产物丙二内酯G是复杂的五味子五味子五味子,具有独特的5/5/7/6/5五环骨架。这篇全文描述了从已知的类固醇内酯简洁地仿生合成丙二内酯G的进展。关键的C19-OH中间体是通过Breslow和Suárez自由基远程CH功能化合成的。Wagner-Meerwein重排随后用于扩大B环。为了转化C10叔醇的构型,用BF 3 ·Et 2催化分子内过氧化物环化O被设计出来。然后按仿生酯交换/ oxa-Michael加成顺序组装5/5融合内酯系统。我们的工作应为拟议的生物合成途径提供实验支持,并促进对丙二内酯G的生物学活性的研究。
更新日期:2020-06-26
中文翻译:

仿丙内酯G†仿生合成的研究进展
天然产物丙二内酯G是复杂的五味子五味子五味子,具有独特的5/5/7/6/5五环骨架。这篇全文描述了从已知的类固醇内酯简洁地仿生合成丙二内酯G的进展。关键的C19-OH中间体是通过Breslow和Suárez自由基远程CH功能化合成的。Wagner-Meerwein重排随后用于扩大B环。为了转化C10叔醇的构型,用BF 3 ·Et 2催化分子内过氧化物环化O被设计出来。然后按仿生酯交换/ oxa-Michael加成顺序组装5/5融合内酯系统。我们的工作应为拟议的生物合成途径提供实验支持,并促进对丙二内酯G的生物学活性的研究。

























 京公网安备 11010802027423号
京公网安备 11010802027423号