当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diels‐Alder reaction mechanisms of substituted chiral anthracene: A theoretical study based on the reaction force and reaction electronic flux
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2020-06-25 , DOI: 10.1002/jcc.26360 Jennifer Paola Hernández Mancera 1 , Francisco Núñez-Zarur 2 , Soledad Gutiérrez-Oliva 3 , Alejandro Toro-Labbé 3 , Ricardo Vivas-Reyes 1, 4
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2020-06-25 , DOI: 10.1002/jcc.26360 Jennifer Paola Hernández Mancera 1 , Francisco Núñez-Zarur 2 , Soledad Gutiérrez-Oliva 3 , Alejandro Toro-Labbé 3 , Ricardo Vivas-Reyes 1, 4
Affiliation
|
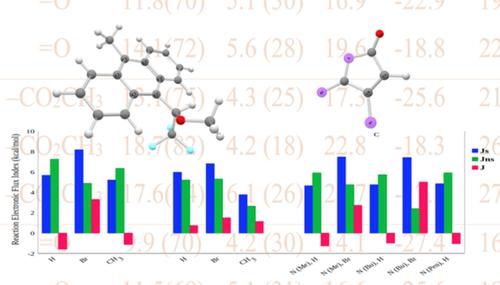
Quantum chemical calculations were used to study the mechanism of Diels‐Alder reactions involving chiral anthracenes as dienes and a series of dienophiles. The reaction force analysis was employed to obtain a detailed scrutiny of the reaction mechanisms, it has been found that thermodynamics and kinetics of the reactions are quite consistent: the lower the activation energy, the lower the reaction energy, thus following the Bell‐Evans‐Polanyi principle. It has been found that activation energies are mostly due to structural rearrangements that in most cases represented more than 70% of the activation energy. Electronic activity mostly due to changes in σ and π bonding were revealed by the reaction electronic flux (REF), this property helps identify whether changes on σ or π bonding drive the reaction. Additionally, new global indexes describing the behavior of the electronic activity were introduced and then used to classify the reactions in terms of the spontaneity of their electronic activity. Local natural bond order electronic population analysis was used to check consistency with global REF through the characterization of specific changes in the electronic density that might be responsible for the activity already detected by the REF. Results show that reactions involving acetoxy lactones are driven by spontaneous electronic activity coming from bond forming/strengthening processes; in the case of maleic anhydrides and maleimides it appears that both spontaneous and non‐spontaneous electronic activity are quite active in driving the reactions.
中文翻译:

取代手性蒽的 Diels-Alder 反应机理:基于反作用力和反应电子通量的理论研究
量子化学计算用于研究涉及手性蒽作为二烯和一系列亲二烯体的 Diels-Alder 反应的机理。采用反作用力分析对反应机理进行了详细审查,发现反应的热力学和动力学非常一致:活化能越低,反应能越低,因此遵循 Bell-Evans-波兰尼原则。已经发现活化能主要是由于结构重排,在大多数情况下占活化能的 70% 以上。反应电子通量 (REF) 揭示了主要由于 σ 和 π 键的变化引起的电子活动,该特性有助于确定 σ 或 π 键的变化是否驱动了反应。此外,引入了描述电子活动行为的新全局指数,然后根据电子活动的自发性对反应进行分类。局部自然键序电子种群分析用于通过表征电子密度的特定变化来检查与全局 REF 的一致性,这些变化可能是由 REF 检测到的活动的原因。结果表明,涉及乙酰氧基内酯的反应是由来自键形成/加强过程的自发电子活动驱动的;在马来酸酐和马来酰亚胺的情况下,似乎自发和非自发的电子活动在驱动反应方面都非常活跃。
更新日期:2020-06-25
中文翻译:

取代手性蒽的 Diels-Alder 反应机理:基于反作用力和反应电子通量的理论研究
量子化学计算用于研究涉及手性蒽作为二烯和一系列亲二烯体的 Diels-Alder 反应的机理。采用反作用力分析对反应机理进行了详细审查,发现反应的热力学和动力学非常一致:活化能越低,反应能越低,因此遵循 Bell-Evans-波兰尼原则。已经发现活化能主要是由于结构重排,在大多数情况下占活化能的 70% 以上。反应电子通量 (REF) 揭示了主要由于 σ 和 π 键的变化引起的电子活动,该特性有助于确定 σ 或 π 键的变化是否驱动了反应。此外,引入了描述电子活动行为的新全局指数,然后根据电子活动的自发性对反应进行分类。局部自然键序电子种群分析用于通过表征电子密度的特定变化来检查与全局 REF 的一致性,这些变化可能是由 REF 检测到的活动的原因。结果表明,涉及乙酰氧基内酯的反应是由来自键形成/加强过程的自发电子活动驱动的;在马来酸酐和马来酰亚胺的情况下,似乎自发和非自发的电子活动在驱动反应方面都非常活跃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号