当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation and Characterization of Dendrimer‐Modified Magnetite Nanoparticles for Adsorption of Humic Acid from Aqueous Solution
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-06-26 , DOI: 10.1002/slct.202000737 Somayeh Beiki 1 , Elham Moniri 2 , Amir Hessam Hassani 1 , Homayon Ahmad Panahi 3
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-06-26 , DOI: 10.1002/slct.202000737 Somayeh Beiki 1 , Elham Moniri 2 , Amir Hessam Hassani 1 , Homayon Ahmad Panahi 3
Affiliation
|
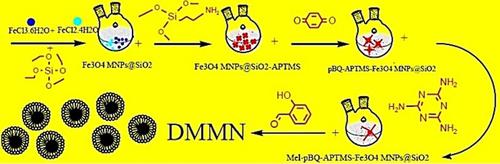
In the present study, innovative Dendrimer‐Modified Magnetite Nanoparticle (DMMN) with core‐shell structures were synthesized as an adsorbent to remove humic acid (HA) from aqueous solution. Using the co‐precipitation technique, super paramagnetic iron nanoparticles were synthesized, and the surface of nanoparticles was functionalized with 3‐aminopropyltrimethoxysilane (APTMS). Then, 10‐generation dendrons were synthesized on the surface of functionalized nanoparticles by polymer grafting method. The sample was characterized by Fourier Transform Infra‐Red (FT‐IR), X‐ray diffraction (XRD), Scanning Electron Microscopy (SEM), Energy Dispersive X‐ray (EDX), and Barrett‐Joyner‐Halenda (BJH) as well as Brunauer‐Emmett‐Teller (BET) analyses, which the surface area of specific DMMN was achieved as 81.37 m2 g−1. Based on the obtained analysis results (SEM, XRD), 90 % of synthesized average nanoparticles diameter is less than 4 nm. Batch experiments were performed under varying operational conditions, namely pH, contact time, initial concentration HA, and adsorbent weight. The optimum adsorbent weight was found to be 0.25 gL−1, while the adsorption process was found to be optimal in the broad pH range of 3–9. The pseudo‐second‐order equation excellently defined the adsorption kinetics when the Langmuir model (R2=0.9967) better fit the adsorption isotherms. The adsorption capacity of DMMN was 103.8 mg HAg−1, that led to 99 % HA adsorption. Moreover, the calculated thermodynamic parameters, including standard enthalpy, entropy, and Gibbs free energy, show the spontaneous and endothermic nature of the adsorption procedure.
中文翻译:

树枝状大分子修饰磁铁矿纳米颗粒的制备及其对腐殖酸的吸附性能
在本研究中,合成了具有核壳结构的创新的树状聚合物修饰的磁铁矿纳米颗粒(DMMN)作为吸附剂,以从水溶液中去除腐殖酸(HA)。使用共沉淀技术,合成了超顺磁性铁纳米粒子,并使用3-氨丙基三甲氧基硅烷(APTMS)对纳米粒子的表面进行了功能化。然后,通过聚合物接枝方法在功能化纳米粒子的表面上合成了10代树突。样品通过傅里叶变换红外(FT-IR),X射线衍射(XRD),扫描电子显微镜(SEM),能量色散X射线(EDX)和Barrett-Joyner-Halenda(BJH)进行表征以及Brunauer-Emmett-Teller(BET)分析,特定DMMN的表面积为81.37 m2 g -1。基于获得的分析结果(SEM,XRD),合成的平均纳米颗粒直径的90%小于4nm。在不同的操作条件下(即pH,接触时间,初始浓度HA和吸附剂重量)进行批量实验。最佳吸附剂重量为0.25 gL -1,而吸附过程在3–9的宽pH范围内最佳。当Langmuir模型(R 2 = 0.9967)更好地拟合吸附等温线时,拟二阶方程很好地定义了吸附动力学。DMMN的吸附容量为103.8 mg HAg -1,导致99%的HA吸附。而且,所计算的热力学参数,包括标准焓,熵和吉布斯自由能,都表明了吸附过程的自发性和吸热性。
更新日期:2020-06-26
中文翻译:

树枝状大分子修饰磁铁矿纳米颗粒的制备及其对腐殖酸的吸附性能
在本研究中,合成了具有核壳结构的创新的树状聚合物修饰的磁铁矿纳米颗粒(DMMN)作为吸附剂,以从水溶液中去除腐殖酸(HA)。使用共沉淀技术,合成了超顺磁性铁纳米粒子,并使用3-氨丙基三甲氧基硅烷(APTMS)对纳米粒子的表面进行了功能化。然后,通过聚合物接枝方法在功能化纳米粒子的表面上合成了10代树突。样品通过傅里叶变换红外(FT-IR),X射线衍射(XRD),扫描电子显微镜(SEM),能量色散X射线(EDX)和Barrett-Joyner-Halenda(BJH)进行表征以及Brunauer-Emmett-Teller(BET)分析,特定DMMN的表面积为81.37 m2 g -1。基于获得的分析结果(SEM,XRD),合成的平均纳米颗粒直径的90%小于4nm。在不同的操作条件下(即pH,接触时间,初始浓度HA和吸附剂重量)进行批量实验。最佳吸附剂重量为0.25 gL -1,而吸附过程在3–9的宽pH范围内最佳。当Langmuir模型(R 2 = 0.9967)更好地拟合吸附等温线时,拟二阶方程很好地定义了吸附动力学。DMMN的吸附容量为103.8 mg HAg -1,导致99%的HA吸附。而且,所计算的热力学参数,包括标准焓,熵和吉布斯自由能,都表明了吸附过程的自发性和吸热性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号