Veterinary Parasitology ( IF 2.6 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.vetpar.2020.109177 Gerhard Bringmann 1 , Shaimaa Fayez 2 , William Shamburger 1 , Doris Feineis 1 , Stanislaw Winiarczyk 3 , Radoslaw Janecki 3 , Łukasz Adaszek 3
|
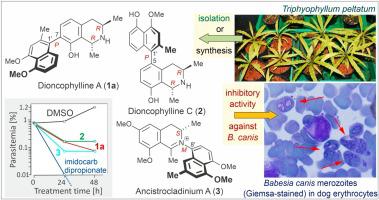
Babesia canis is the predominant and clinically relevant canine Babesia species in Europe. Transmitted by vector ticks, the parasite enters red blood cells and induces a severe, potentially fatal hemolytic anemia. Here, we report on the antibabesial activities of three extracts of the West African tropical plant species Triphyophyllum peltatum (Dioncophyllaceae) and Ancistrocladus abbreviatus (Ancistrocladaceae) and of 13 genuine naphthylisoquinoline alkaloids isolated thereof. Two of the extracts and eight of the alkaloids were found to display strong activities against Babesia canis in vitro. Among the most potent compounds were the C,C-coupled dioncophyllines A (1a) and C (2) and the N,C-linked alkaloids ancistrocladium A (3) and B (4), with half-maximum inhibition concentration (IC50) values of 0.48 μM for 1a, 0.85 μM for 2, 1.90 μM for 3, and 1.23 μM for 4. Structure-activity relationship (SAR) studies on a small library of related genuine analogs and non-natural synthetic derivatives of 1a and 2 revealed the likewise naturally occurring alkaloid N-methyl-7-epi-dioncophylline A (6b) to be the most potent (IC50, 0.14 μM) among the investigated compounds. Although none of the tested naphthylisoquinolines showed 100 % inhibition of parasite infection – as displayed by imidocarb dipropionate (IC50, 0.07 μM), which was used as a positive control – the antibabesial potential of the dioncophyllines A (1a) and C (2) and related compounds such as 6b, its atropo-diastereomer 6a (IC50, 1.45 μM), and 8-O-(p-nitrobenzyl)dioncophylline A (14) (IC50, 0.82 μM) is to be considered as high. The SAR results showed that N-methylation and axial chirality exert a strong impact on the antibabasial activities of the naphthylisoquinolines presented here, whereas dimerization, as in jozimine A2 (5) (IC50, 140 μM), leads to a significant decrease of activity against B. canis. Alkaloids displaying good to high activities against B. canis like the dioncophyllines 1a, 2, 6a, and 6b were found to cause only a small degree of hemolysis (< 0.7 %), whereas compounds with moderate to weak antibabesial activities such as 6-O-methyl-4′-O-demethylancistrocladine (15a) (IC50, 14.0 μM) and its atropo-diastereomer 6-O-methyl-4′-O-demethylhamatine (15b) (IC50, 830 μM) caused a high degree of hemolysis (7.3 % for 15a and 11.2 % for 15b). In this respect, the most effective anti-Babesia naphthylisoquinolines are also the safest ones.
中文翻译:

萘异喹啉碱生物碱及其合成类似物可作为体外抗犬贝贝斯病的有效新抑制剂。
巴贝斯犬是欧洲主要和与临床相关的犬贝贝斯菌种。通过媒介tick传播,该寄生虫进入红细胞并引起严重的,可能致命的溶血性贫血。在这里,我们报告西非热带植物物种Triphyophyllum peltatum(Dioncophyllaceae)和Ancistrocladus abbreviatus(Ancistrocladaceae)的三种提取物及其分离的13种真正的萘基异喹啉类生物碱的抑菌活性。发现两种提取物和八种生物碱在体外对犬贝贝氏菌具有较强的活性。其中最有效的化合物是C,C偶联的二异茶碱A(1a)和C(2)和N,C联结的生物碱Antrotrocladium A(3)和B(4),半最大抑制浓度(IC 50)值分别为1a 0.48μM,2 0.85μM,3 1.90μM和1.23μM为4。结构-活性关系(SAR)对相关真正类似物和非天然合成的衍生物的一小库研究1A和2揭示了同样天然存在的生物碱ñ -甲基-7-外延-dioncophylline A(图6b)是最有效的(IC 50(0.14μM)。虽然没有任何测试naphthylisoquinolines的显示寄生虫感染的100%的抑制-如由双咪苯脲二丙酸(IC显示50的dioncophyllines A(的antibabesial电位- ,0.07μM),其被用作阳性对照1A)和C(2)和相关的化合物,如图6b,其atropo非对映体6A(IC 50,μM1.45),和8- ö - (p -硝基苄基)dioncophylline A(14)(IC 50,μM0.82)被认为是高的。特区结果表明,ñ-methylation和轴向手性施加在naphthylisoquinolines的antibabasial活动的强烈冲击这里介绍的,而二聚化,如在jozimine甲2(5)(IC 50,μM140),导致活性的针对显著降低B.犬。生物碱显示出良好的高活性抗B. CANIS像dioncophyllines 1A,2,图6a和6b中被发现只引起很小的程度的溶血(<0.7%)的,而化合物具有中度至弱antibabesial活动,如6- ö -甲基-4'- O-去甲基顺氯cladine(15a)(IC 50,μM14.0)及其atropo非对映体6- O-甲基-4' - Ö -demethylhamatine(15B)(IC 50,μM830)引起的高度溶血(7.3%的15A为和11.2%15b)。在这方面,最有效的抗贝贝氏菌萘异喹啉也是最安全的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号