当前位置:
X-MOL 学术
›
Mater. Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface potential and roughness controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration
Materials & Design ( IF 8.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.matdes.2020.108915 Sara Metwally , Sara Ferraris , Silvia Spriano , Zuzanna J. Krysiak , Łukasz Kaniuk , Mateusz M. Marzec , Sung Kyun Kim , Piotr K. Szewczyk , Adam Gruszczyński , Magdalena Wytrwal-Sarna , Joanna E. Karbowniczek , Andrzej Bernasik , Sohini Kar-Narayan , Urszula Stachewicz
Materials & Design ( IF 8.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.matdes.2020.108915 Sara Metwally , Sara Ferraris , Silvia Spriano , Zuzanna J. Krysiak , Łukasz Kaniuk , Mateusz M. Marzec , Sung Kyun Kim , Piotr K. Szewczyk , Adam Gruszczyński , Magdalena Wytrwal-Sarna , Joanna E. Karbowniczek , Andrzej Bernasik , Sohini Kar-Narayan , Urszula Stachewicz
|
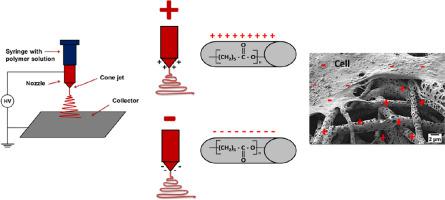
Abstract Surface potential of biomaterials is a key factor regulating cell responses, driving their adhesion and signaling in tissue regeneration. In this study we compared the surface and zeta potential of smooth and porous electrospun polycaprolactone (PCL) fibers, as well as PCL films, to evaluate their significance in bone regeneration. The ’ surface potential of the fibers was controlled by applying positive and negative voltage polarities during the electrospinning. The surface properties of the different PCL fibers and films were measured using X-ray photoelectron spectroscopy (XPS) and Kelvin probe force microscopy (KPFM), and the zeta potential was measured using the electrokinetic technique. The effect of surface potential on the morphology of bone cells was examined using advanced microcopy, including 3D reconstruction based on a scanning electron microscope with a focused ion beam (FIB-SEM). Initial cell adhesion and collagen formation were studied using fluorescence microscopy and Sirius Red assay respectively, while calcium mineralization was confirmed with energy-dispersive x-ray (EDX) and Alzarin Red staining. These studies revealed that cell adhesion is driven by both the surface potential and morphology of PCL fibers. Furthermore, the ability to tune the surface potential of electrospun PCL scaffolds provides an essential electrostatic handle to enhance cell-material interaction and cellular activity, leading to controllable morphological changes.
中文翻译:

用于骨再生的电纺 PCL 纤维中的表面电位和粗糙度控制细胞粘附和胶原形成
摘要 生物材料的表面电位是调节细胞反应的关键因素,驱动它们在组织再生中的粘附和信号传导。在这项研究中,我们比较了光滑多孔的电纺聚己内酯 (PCL) 纤维以及 PCL 薄膜的表面和 zeta 电位,以评估它们在骨再生中的重要性。通过在静电纺丝过程中施加正负电压极性来控制纤维的表面电位。使用 X 射线光电子能谱 (XPS) 和开尔文探针力显微镜 (KPFM) 测量不同 PCL 纤维和薄膜的表面特性,并使用电动技术测量 zeta 电位。使用先进的显微镜检查表面电位对骨细胞形态的影响,包括基于具有聚焦离子束的扫描电子显微镜 (FIB-SEM) 的 3D 重建。分别使用荧光显微镜和天狼星红测定研究初始细胞粘附和胶原形成,而钙矿化则通过能量色散 X 射线 (EDX) 和 Alzarin Red 染色证实。这些研究表明,细胞粘附是由 PCL 纤维的表面电位和形态驱动的。此外,调节电纺 PCL 支架表面电位的能力为增强细胞材料相互作用和细胞活性提供了必不可少的静电手柄,从而导致可控的形态变化。而通过能量色散 X 射线 (EDX) 和 Alzarin Red 染色证实钙矿化。这些研究表明,细胞粘附是由 PCL 纤维的表面电位和形态驱动的。此外,调节电纺 PCL 支架表面电位的能力为增强细胞材料相互作用和细胞活性提供了必不可少的静电手柄,从而导致可控的形态变化。而通过能量色散 X 射线 (EDX) 和 Alzarin Red 染色证实钙矿化。这些研究表明,细胞粘附是由 PCL 纤维的表面电位和形态驱动的。此外,调节电纺 PCL 支架表面电位的能力为增强细胞材料相互作用和细胞活性提供了必不可少的静电手柄,从而导致可控的形态变化。
更新日期:2020-09-01
中文翻译:

用于骨再生的电纺 PCL 纤维中的表面电位和粗糙度控制细胞粘附和胶原形成
摘要 生物材料的表面电位是调节细胞反应的关键因素,驱动它们在组织再生中的粘附和信号传导。在这项研究中,我们比较了光滑多孔的电纺聚己内酯 (PCL) 纤维以及 PCL 薄膜的表面和 zeta 电位,以评估它们在骨再生中的重要性。通过在静电纺丝过程中施加正负电压极性来控制纤维的表面电位。使用 X 射线光电子能谱 (XPS) 和开尔文探针力显微镜 (KPFM) 测量不同 PCL 纤维和薄膜的表面特性,并使用电动技术测量 zeta 电位。使用先进的显微镜检查表面电位对骨细胞形态的影响,包括基于具有聚焦离子束的扫描电子显微镜 (FIB-SEM) 的 3D 重建。分别使用荧光显微镜和天狼星红测定研究初始细胞粘附和胶原形成,而钙矿化则通过能量色散 X 射线 (EDX) 和 Alzarin Red 染色证实。这些研究表明,细胞粘附是由 PCL 纤维的表面电位和形态驱动的。此外,调节电纺 PCL 支架表面电位的能力为增强细胞材料相互作用和细胞活性提供了必不可少的静电手柄,从而导致可控的形态变化。而通过能量色散 X 射线 (EDX) 和 Alzarin Red 染色证实钙矿化。这些研究表明,细胞粘附是由 PCL 纤维的表面电位和形态驱动的。此外,调节电纺 PCL 支架表面电位的能力为增强细胞材料相互作用和细胞活性提供了必不可少的静电手柄,从而导致可控的形态变化。而通过能量色散 X 射线 (EDX) 和 Alzarin Red 染色证实钙矿化。这些研究表明,细胞粘附是由 PCL 纤维的表面电位和形态驱动的。此外,调节电纺 PCL 支架表面电位的能力为增强细胞材料相互作用和细胞活性提供了必不可少的静电手柄,从而导致可控的形态变化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号