Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.jmgm.2020.107648 Nguyen Thi Mai 1 , Ngo Thi Lan 2 , Thien Y Vu 3 , Phuong Thi Mai Duong 4 , Nguyen Thanh Tung 2 , Huong Thi Thu Phung 5
|
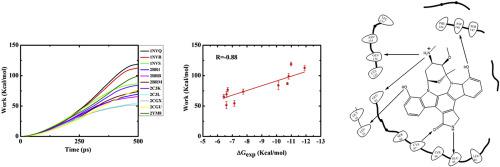
Checkpoint kinase 1 (CHK1) is a serine/threonine-protein kinase that is involved in cell cycle regulation in eukaryotes. Inhibition of CHK1 is thus considered as a promising approach in cancer therapy. In this study, the fast pulling of ligand (FPL) process was applied to predict the relative binding affinities of CHK1 inhibitors using non-equilibrium molecular dynamics (MD) simulations. The work of external harmonic forces to pull the ligand out of the binding cavity strongly correlated with the experimental binding affinity of CHK1 inhibitors with the correlation coefficient of R = −0.88 and an overall root mean square error (RMSE) of 0.99 kcal/mol. The data indicate that the FPL method is highly accurate in predicting the relative binding free energies of CHK1 inhibitors with an affordable CPU time. A new set of molecules were designed based on the molecular modeling of interactions between the known inhibitor and CHK1 as inhibitory candidates. Molecular docking and FPL results exhibited that the binding affinities of developed ligands were similar to the known inhibitor in interaction with the catalytic site of CHK1, producing very potential CHK1 inhibitors of that the inhibitory activities should be further evaluated in vitro.
中文翻译:

通过非平衡MD模拟估算检查点激酶1的配体结合自由能。
Checkpoint激酶1(CHK1)是一种丝氨酸/苏氨酸蛋白激酶,参与真核生物的细胞周期调控。因此,抑制CHK1被认为是癌症治疗中的一种有前途的方法。在这项研究中,使用非平衡分子动力学(MD)模拟将配体的快速拉动(FPL)过程用于预测CHK1抑制剂的相对结合亲和力。外部谐波将配体从结合腔中拉出的功与CHK1抑制剂的实验结合亲和力密切相关,相关系数R = -0.88,总均方根误差(RMSE)为0.99 kcal / mol。数据表明,FPL方法在以可承受的CPU时间预测CHK1抑制剂的相对结合自由能方面非常准确。基于已知抑制剂与作为抑制候选物的CHK1之间相互作用的分子模型,设计了一组新的分子。分子对接和FPL结果表明,已开发的配体在与CHK1催化位点相互作用时的结合亲和力与已知抑制剂相似,产生了非常有潜力的CHK1抑制剂,其抑制活性应在体外进一步评估。


























 京公网安备 11010802027423号
京公网安备 11010802027423号