Electrochimica Acta ( IF 6.6 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.electacta.2020.136657 Bohyeon Kim , Gautam Das , Bang Ju Park , Dal Ho Lee , Hyon Hee Yoon
|
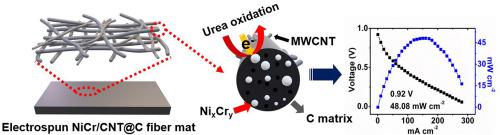
A highly porous free-standing NiCr-bimetallic catalyst is synthesized by electrospinning and carbonization and is applied as an anode in a direct urea/H2O2 fuel cell for electro-oxidation of urea. It is observed that the morphology of the calcined NiCr catalyst, which has a higher specific surface area, is identical to that of the electrospun NiCr catalyst fiber. Cr-doping at 40% (Cr/Ni) significantly increases the oxidation peak current of the urea oxidation reaction. The addition of carbon nanotubes also considerably enhances the catalytic activity. A direct urea/H2O2 fuel cell, which utilizes the synthesized catalyst (NiCr-CNT@C) as a free-standing anode, exhibits excellent performance with a maximum power density of 48.1 mW cm−2 and an open-circuit voltage of 0.92 V at 80 °C. Thus, the highly porous free-standing NiCr-CNT@C catalyst mat can be employed as an efficient anode material for urea oxidation in urea fuel cells.
中文翻译:

通过静电纺丝的独立式NiCr-CNT @ C阳极毡,用于高性能尿素/ H2O2燃料电池
通过电纺丝和碳化合成高度多孔的独立式NiCr双金属催化剂,并将其用作直接尿素/ H 2 O 2燃料电池中的阳极,用于尿素的电氧化。观察到,具有较高比表面积的煅烧NiCr催化剂的形态与电纺NiCr催化剂纤维的形态相同。以40%(Cr / Ni)掺杂Cr会显着增加尿素氧化反应的氧化峰值电流。碳纳米管的加入也大大增强了催化活性。利用合成催化剂(NiCr-CNT @ C)作为独立式阳极的直接尿素/ H 2 O 2燃料电池具有出色的性能,最大功率密度为48.1 mW cm-2和80°C下的开路电压为0.92V。因此,高度多孔的独立式NiCr-CNT @ C催化剂垫可用作尿素燃料电池中尿素氧化的有效阳极材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号