Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.bioorg.2020.104047 Remah Sobhy 1 , Ibrahim Khalifa 2 , Hongshan Liang 3 , Bin Li 4
|
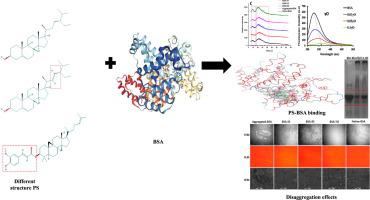
Discovering small molecules with protein-disaggregation effects is recently needed. For the first time, we intensely studied the anti-amyloidogenic effects of 3 structurally different phytosterols (PS), namely stigmasterol, β-sitosterol, and γ-oryzanol, on bovine serum albumin (BSA) under aggregations-promoting conditions using multispectral, microstructure, and molecular docking methods. Results found that PS dose- and structure- dependently inhibited BSA-aggregations under the glycation conditions through separating BSA-peak size, quenching Tryptophan-intensity, altering BSA-hydrophobicity, and microstructural declining the aggregates of glycated-BSA. Throughout the underlying mechanism beyond its disaggregation effects, PS reformed cross-β-sheet structure, SDS-PAGE-bands, and XRD-peaks of glycated-BSA aggregates. Most importantly, PS were found to bind with some lysyl and arginine glycation sites of BSA, specifically Lys114, Lys116, Lys136, Lys431, Arg427, and Arg185, via Hydrogen-bonding with their –OH-groups and pi-pi interactions of their steroid core. Taken together, the current results unleash that PS could restrict BSA-aggregations under the glycation conditions and their subsequent changes, which can assist in the design of reasonable therapeutics.
中文翻译:

植物固醇通过与糖基化位点相互作用并改变其二级结构元素,在糖基化条件下分解牛血清白蛋白。
最近需要发现具有蛋白质分解作用的小分子。我们首次使用多光谱,微观结构深入研究了在聚集促进条件下三种结构不同的植物甾醇(PS),即豆甾醇,β-谷甾醇和γ-谷维素对牛血清白蛋白(BSA)的抗淀粉样蛋白作用。 ,以及分子对接方法。结果发现,在糖基化条件下,PS通过分离BSA峰大小,猝灭色氨酸强度,改变BSA疏水性和微观结构降低糖化BSA聚集体,在糖基化条件下抑制了BSA聚集。在其分解作用之外的整个潜在机制中,PS重整了糖基化BSA聚集体的交叉β-折叠结构,SDS-PAGE带和XRD峰。最重要的是,发现PS通过与它们的–OH-基团和甾体核心的pi-pi相互作用进行氢键结合,与BSA的某些赖氨酰和精氨酸糖基化位点结合,特别是Lys114,Lys116,Lys136,Lys431,Arg427和Arg185。综上所述,当前结果释放出PS可以在糖基化条件及其后续变化下限制BSA聚集,从而有助于设计合理的治疗药物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号