Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.bbamcr.2020.118786 Paul A Wadsworth 1 , Aditya K Singh 2 , Nghi Nguyen 3 , Nolan M Dvorak 2 , Cynthia M Tapia 2 , William K Russell 4 , Clifford Stephan 3 , Fernanda Laezza 5
|
Background
Protein interactions between voltage-gated sodium (Nav) channels and accessory proteins play an essential role in neuronal firing and plasticity. However, a surprisingly limited number of kinases have been identified as regulators of these molecular complexes. We hypothesized that numerous as-of-yet unidentified kinases indirectly regulate the Nav channel via modulation of the intracellular fibroblast growth factor 14 (FGF14), an accessory protein with numerous unexplored phosphomotifs and required for channel function in neurons.
Methods
Here we present results from an in-cell high-throughput screening (HTS) against the FGF14: Nav1.6 complex using >3000 diverse compounds targeting an extensive range of signaling pathways. Regulation by top kinase targets was then explored using in vitro phosphorylation, biophysics, mass-spectrometry and patch-clamp electrophysiology.
Results
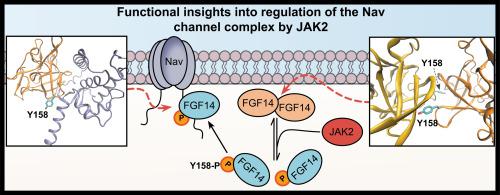
Compounds targeting Janus kinase 2 (JAK2) were over-represented among HTS hits. Phosphomotif scans supported by mass spectrometry revealed FGF14Y158, a site previously shown to mediate both FGF14 homodimerization and interactions with Nav1.6, as a JAK2 phosphorylation site. Following inhibition of JAK2, FGF14 homodimerization increased in a manner directly inverse to FGF14:Nav1.6 complex formation, but not in the presence of the FGF14Y158A mutant. Patch-clamp electrophysiology revealed that through Y158, JAK2 controls FGF14-dependent modulation of Nav1.6 channels. In hippocampal CA1 pyramidal neurons, the JAK2 inhibitor Fedratinib reduced firing by a mechanism that is dependent upon expression of FGF14.
Conclusions
These studies point toward a novel mechanism by which levels of JAK2 in neurons could directly influence firing and plasticity by controlling the FGF14 dimerization equilibrium, and thereby the availability of monomeric species for interaction with Nav1.6.
中文翻译:

JAK2 通过 FGF14Y158 磷酸化调节 Nav1.6 通道功能。
背景
电压门控钠 (Nav) 通道和辅助蛋白之间的蛋白质相互作用在神经元放电和可塑性中发挥着重要作用。然而,令人惊讶的是,数量有限的激酶已被确定为这些分子复合物的调节剂。我们假设许多尚未鉴定的激酶通过调节细胞内成纤维细胞生长因子 14 (FGF14) 来间接调节 Nav 通道,FGF14 是一种具有许多未开发的磷酸基序的辅助蛋白,是神经元通道功能所必需的。
方法
在这里,我们展示了针对 FGF14: Nav1.6 复合物的细胞内高通量筛选 (HTS) 结果,使用超过 3000 种针对广泛信号通路的不同化合物。然后利用体外磷酸化、生物物理学、质谱和膜片钳电生理学探索顶级激酶靶点的调节。
结果
HTS 命中中针对 Janus 激酶 2 (JAK2) 的化合物比例过高。质谱支持的 Phosphomotif 扫描显示 FGF14 Y158是一个 JAK2 磷酸化位点,之前显示该位点介导 FGF14 同二聚化以及与 Nav1.6 的相互作用。抑制 JAK2 后,FGF14 同二聚化以与 FGF14:Nav1.6 复合物形成直接相反的方式增加,但在 FGF14 Y158A突变体存在时则不然。膜片钳电生理学显示,JAK2 通过 Y158 控制 Nav1.6 通道的 FGF14 依赖性调节。在海马 CA1 锥体神经元中,JAK2 抑制剂 Fedratinib 通过依赖 FGF14 表达的机制减少放电。
结论
这些研究指出了一种新的机制,通过该机制,神经元中的 JAK2 水平可以通过控制 FGF14 二聚化平衡来直接影响放电和可塑性,从而影响单体物种与 Nav1.6 相互作用的可用性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号