当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pnictogen-bonding catalysis: brevetoxin-type polyether cyclizations
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-25 , DOI: 10.1039/d0sc02551h Andrea Gini 1, 2, 3, 4 , Miguel Paraja 1, 2, 3, 4 , Bartomeu Galmés 5, 6, 7, 8 , Celine Besnard 1, 2, 3, 4 , Amalia I. Poblador-Bahamonde 1, 2, 3, 4 , Naomi Sakai 1, 2, 3, 4 , Antonio Frontera 5, 6, 7, 8 , Stefan Matile 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-25 , DOI: 10.1039/d0sc02551h Andrea Gini 1, 2, 3, 4 , Miguel Paraja 1, 2, 3, 4 , Bartomeu Galmés 5, 6, 7, 8 , Celine Besnard 1, 2, 3, 4 , Amalia I. Poblador-Bahamonde 1, 2, 3, 4 , Naomi Sakai 1, 2, 3, 4 , Antonio Frontera 5, 6, 7, 8 , Stefan Matile 1, 2, 3, 4
Affiliation
|
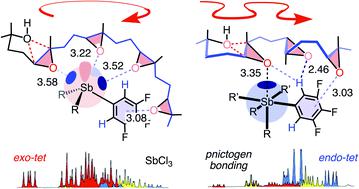
Pnictogen-bond donors are attractive for use in catalysis because of deep σ holes, high multivalency, rich hypervalency, and chiral binding pockets. We here report natural product inspired epoxide-opening polyether cyclizations catalyzed by fluoroarylated Sb(V) > Sb(III) > Bi > Sn > Ge. The distinctive characteristic found for pnictogen-bonding catalysis is the breaking of the Baldwin rules, that is selective endo cyclization into the trans-fused ladder oligomers known from the brevetoxins. Moreover, tris(3,4,5-trifluorophenyl)stibines and their hypervalent stiborane catecholates afford different anti-Baldwin stereoselectivity. Lewis (SbCl3), Brønsted (AcOH) and π acids fail to provide similar access to these forbidden rings. Like hydrogen-bonding catalysis differs from Brønsted acid catalysis, pnictogen-bonding catalysis thus emerges as the supramolecular counterpart of covalent Lewis acid catalysis.
中文翻译:

光子键催化:短毒素型聚醚环化
光子键供体由于具有较大的σ孔,高多价,丰富的高价和手性结合口袋,因此在催化中具有吸引力。我们在此报告了由氟代芳基化的Sb(V)> Sb(III)> Bi> Sn> Ge催化的天然产物启发的开环聚醚环化反应。为氮族元素接合催化的独特特性发现的是的鲍德温规则的断裂,即选择性内切环化到反式从brevetoxins已知-融合梯低聚物。此外,三(3,4,5-三氟苯基)stibines及其高价stiborane儿茶酚酯具有不同的抗-Baldwin立体选择性。刘易斯(SbCl 3),布朗斯台德(AcOH)和π酸无法为这些禁环提供类似的通道。就像氢键催化不同于布朗斯台德酸催化一样,光子键催化也因此作为共价路易斯酸催化的超分子形式出现。
更新日期:2020-07-15
中文翻译:

光子键催化:短毒素型聚醚环化
光子键供体由于具有较大的σ孔,高多价,丰富的高价和手性结合口袋,因此在催化中具有吸引力。我们在此报告了由氟代芳基化的Sb(V)> Sb(III)> Bi> Sn> Ge催化的天然产物启发的开环聚醚环化反应。为氮族元素接合催化的独特特性发现的是的鲍德温规则的断裂,即选择性内切环化到反式从brevetoxins已知-融合梯低聚物。此外,三(3,4,5-三氟苯基)stibines及其高价stiborane儿茶酚酯具有不同的抗-Baldwin立体选择性。刘易斯(SbCl 3),布朗斯台德(AcOH)和π酸无法为这些禁环提供类似的通道。就像氢键催化不同于布朗斯台德酸催化一样,光子键催化也因此作为共价路易斯酸催化的超分子形式出现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号