当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of a more potent anticancer agent than C4‐benzazole 1,8‐naphthalimide derivatives against murine melanoma
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-06-24 , DOI: 10.1002/jccs.202000019 Chi‐Hua Tung, Yen‐Ta Lu, Wei‐Ting Kao, Jen‐Wei Liu, Yi‐Hsuan Lai, Shinn‐Jong Jiang, Hao‐Ping Chen, Tzenge‐Lien Shih
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-06-24 , DOI: 10.1002/jccs.202000019 Chi‐Hua Tung, Yen‐Ta Lu, Wei‐Ting Kao, Jen‐Wei Liu, Yi‐Hsuan Lai, Shinn‐Jong Jiang, Hao‐Ping Chen, Tzenge‐Lien Shih
|
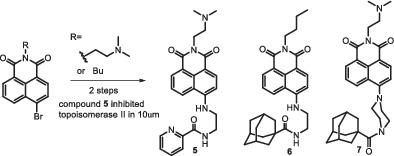
Three novel naphthalimide‐based derivatives were synthesized and tested in vitro as anticancer agents. Our previous report of the C4‐benzazole 1,8‐naphthalimide derivatives showed good inhibition against murine melanoma. We aimed to synthesize more potent agents and found that compound 5 reported in this article behaved 5‐ to 10‐fold potency than our previous best results. The unique structure of compound 5 consisted of a naphthalimide framework in which C4 position was linked with an ethylenediamine group where the amino group was coupled with a 2‐piconic acid moiety. Compound 5 exhibited the most potent inhibitory activity toward human DNA topoisomerase II proteins with IC50 value (2.6 ± 0.1 μM) against murine B16F10 melanoma cells among the three target compounds synthesized in this study. In accordance with this finding, the results of molecular docking also revealed that compound 5 has the highest affinity with human DNA topoisomerase II among the selected compounds. Compound 5 , therefore, has high potential for becoming a lead compound.
中文翻译:

发现比C4-苯并唑1,8-萘二甲酰亚胺衍生物更有效的抗鼠黑色素瘤抗癌药
合成了三种基于萘二甲酰亚胺的新型衍生物,并作为抗癌剂进行了体外测试。我们先前关于C4-苯并唑1,8-萘二甲酰亚胺衍生物的报告显示出对鼠黑色素瘤的良好抑制作用。我们旨在合成更有效的药物,并发现本文报道的化合物5的效能比我们以前的最佳结果高5至10倍。化合物5的独特结构由萘二甲酰亚胺骨架组成,其中C4位置与乙二胺基连接,其中氨基与2-吡啶甲酸部分偶联。化合物5对人DNA拓扑异构酶II蛋白的IC 50表现出最强的抑制活性在这项研究中合成的三种目标化合物中,针对鼠B16F10黑色素瘤细胞的最大抗药性(2.6±0.1μM)。根据这一发现,分子对接的结果还表明,在所选化合物中,化合物5与人DNA拓扑异构酶II的亲和力最高。因此,化合物5具有成为前导化合物的高潜力。
更新日期:2020-07-13
中文翻译:

发现比C4-苯并唑1,8-萘二甲酰亚胺衍生物更有效的抗鼠黑色素瘤抗癌药
合成了三种基于萘二甲酰亚胺的新型衍生物,并作为抗癌剂进行了体外测试。我们先前关于C4-苯并唑1,8-萘二甲酰亚胺衍生物的报告显示出对鼠黑色素瘤的良好抑制作用。我们旨在合成更有效的药物,并发现本文报道的化合物5的效能比我们以前的最佳结果高5至10倍。化合物5的独特结构由萘二甲酰亚胺骨架组成,其中C4位置与乙二胺基连接,其中氨基与2-吡啶甲酸部分偶联。化合物5对人DNA拓扑异构酶II蛋白的IC 50表现出最强的抑制活性在这项研究中合成的三种目标化合物中,针对鼠B16F10黑色素瘤细胞的最大抗药性(2.6±0.1μM)。根据这一发现,分子对接的结果还表明,在所选化合物中,化合物5与人DNA拓扑异构酶II的亲和力最高。因此,化合物5具有成为前导化合物的高潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号