当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Versatile Reaction Patterns of Phosphanylhydrosilylalkyne with B(C6F5)3: A Remarkable Group Substitution Effect
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-06-25 , DOI: 10.1002/ejic.202000506 Yanting Huang, Wenjun Jiang, Xin Xi, Yan Li, Xiaoping Wang, Ming‐Chung Yang, Zheng‐Feng Zhang, Ming‐Der Su, Hongping Zhu
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-06-25 , DOI: 10.1002/ejic.202000506 Yanting Huang, Wenjun Jiang, Xin Xi, Yan Li, Xiaoping Wang, Ming‐Chung Yang, Zheng‐Feng Zhang, Ming‐Der Su, Hongping Zhu
|
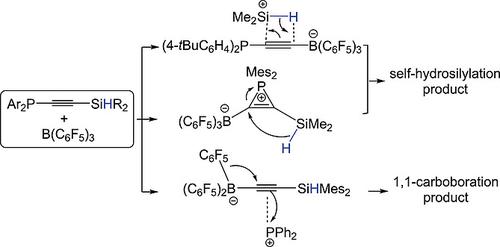
Phosphanylhydrosilylalkynes R2HSiCCPAr2 (R,Ar: Me,4‐tBuC6H4 1, Me,Mes 2, Mes,Ph 3; Mes = 2,4,6‐Me3C6H2) were prepared and the reactions with B(C6F5)3 were studied. Reaction of 1 and B(C6F5)3 produced E‐alkene {(E)‐(C6F5)3BCHC[P(4‐tBuC6H4)2]SiMe2}2 (4) and that of 2 and B(C6F5)3 yielded Z‐alkene (Z)‐(C6F5)2BCHC(PMes2)SiMe2(C6F5) (5). The former is proposed to go through a key [Me2HSi]+ for function while the latter via the phosphacyclopropene intermediate, both of which are a result by self‐hydrosilylation. Reaction of 3 and B(C6F5)3 generated at room temperature a P→B coordination compound Mes2HSiCCP(Ph2)B(C6F5)3 (6) and at 100 °C the 1,1‐carboboration E‐alkene (E)‐Mes2HSi(Ph2P)CC(C6F5)B(C6F5)2 (7). Kinetic study and DFT calculations were accomplished for reaction of 2 and B(C6F5)3 to 5. The mechanisms of these reactions have been discussed. The reactions of the P/Si+ LPs Me2Si(Ph2P)CCHB(C6F5)3}2 (3a) and 4 were also investigated. Compound 4 disassociated H2O into {(E)‐(C6F5)3BCHC[PH‐4‐tBuC6H4)2]Si(Me2)}2(µ‐O) (8) and (E)‐(C6F5)3BCHC[PH(4‐tBu‐C6H4)2]Si(Me2)O(HNC5H5) (9). Compound 3a reacted with tBuNCO by [3+2] dipolar cycloaddition to give a C2OPSi‐heterocycle [(C6F5)3BHC]CSi(Me2)P(Ph2)OC(NtBu) (10). Furthermore, 4 reacted with tBuNCO and then H2O to afford (E)‐(F5C6)3BHCC[P(4‐tBuC6H4)2C(O)NHtBu][Si(Me2)OH(NC5H5)] (11) through a C2OPSi‐heterocycle intermediate followed by the H2O‐disassociation under the C2OPSi‐ring opening.
中文翻译:

膦酰基氢甲硅烷基炔与B(C6F5)3的多功能反应模式:显着的基团取代作用。
制备了膦酰基氢硅烷基炔烃R 2 HSiCCPAr 2(R,Ar:Me,4- t BuC 6 H 4 1,Me,Mes 2,Mes,Ph 3 ; Mes = 2,4,6-Me 3 C 6 H 2),研究了与B(C 6 F 5)3的反应。的反应1和B(C 6 ˚F 5)3产生È -烯烃{(ë(C - )6 ˚F 5)3 BCHC [P(4-吨BUC6 ħ 4)2 ]森达2 } 2(4)和的2和B(C 6 ˚F 5)3,得到Ž -烯烃(Ž) - (C 6 ˚F 5)2 BCHC(机动设备2)森达2(C 6 F 5)(5)。建议前者通过密钥[Me 2 HSi] +后者通过磷环丙烯中间体发挥作用,两者都是自氢硅烷化的结果。的反应3和B(C 6 ˚F 5)3在室温下为P→乙配位化合物生成的Mes 2 HSiCCP(PH 2)B(C 6 ˚F 5)3(6),并在100℃下的1,1-二碳硼化E-烯烃(E)-Mes 2 HSi(Ph 2 P)CC(C 6 F 5)B(C 6 F 5)2(7)。对2和B(C 6 F 5)3至5的反应进行了动力学研究和DFT计算。已经讨论了这些反应的机理。还研究了P / Si + LPs Me 2 Si(Ph 2 P)CCHB(C 6 F 5)3 } 2(3a)和4的反应。化合物4将H 2 O分解为{(E)‐(C 6 F 5)3 BCHC [PH‐4‐ t BuC6 H 4)2 ] Si(Me 2)} 2(µ- O)(8)和(E)-(C 6 F 5)3 BCHC [PH(4 - t Bu‐C 6 H 4)2 ] Si (Me 2)O(HNC 5 H 5)(9)。化合物3a通过[3 + 2]偶极环加成与t BuNCO反应,得到C 2 OPSi-杂环[(C 6 F 5)3 BHC] CSi(Me 2)P(Ph 2)OC(N t Bu)(10)。此外,4与t BuNCO反应,然后与H 2 O反应,得到(E)-(F 5 C 6)3 BHCC [P(4- t BuC 6 H 4)2 C(O)NH t Bu] [Si(Me 2)OH(NC 5 H 5)](11)通过C 2 OPSi杂环中间体,然后在C 2 OPSi环开口下进行H 2 O分解。
更新日期:2020-06-25
中文翻译:

膦酰基氢甲硅烷基炔与B(C6F5)3的多功能反应模式:显着的基团取代作用。
制备了膦酰基氢硅烷基炔烃R 2 HSiCCPAr 2(R,Ar:Me,4- t BuC 6 H 4 1,Me,Mes 2,Mes,Ph 3 ; Mes = 2,4,6-Me 3 C 6 H 2),研究了与B(C 6 F 5)3的反应。的反应1和B(C 6 ˚F 5)3产生È -烯烃{(ë(C - )6 ˚F 5)3 BCHC [P(4-吨BUC6 ħ 4)2 ]森达2 } 2(4)和的2和B(C 6 ˚F 5)3,得到Ž -烯烃(Ž) - (C 6 ˚F 5)2 BCHC(机动设备2)森达2(C 6 F 5)(5)。建议前者通过密钥[Me 2 HSi] +后者通过磷环丙烯中间体发挥作用,两者都是自氢硅烷化的结果。的反应3和B(C 6 ˚F 5)3在室温下为P→乙配位化合物生成的Mes 2 HSiCCP(PH 2)B(C 6 ˚F 5)3(6),并在100℃下的1,1-二碳硼化E-烯烃(E)-Mes 2 HSi(Ph 2 P)CC(C 6 F 5)B(C 6 F 5)2(7)。对2和B(C 6 F 5)3至5的反应进行了动力学研究和DFT计算。已经讨论了这些反应的机理。还研究了P / Si + LPs Me 2 Si(Ph 2 P)CCHB(C 6 F 5)3 } 2(3a)和4的反应。化合物4将H 2 O分解为{(E)‐(C 6 F 5)3 BCHC [PH‐4‐ t BuC6 H 4)2 ] Si(Me 2)} 2(µ- O)(8)和(E)-(C 6 F 5)3 BCHC [PH(4 - t Bu‐C 6 H 4)2 ] Si (Me 2)O(HNC 5 H 5)(9)。化合物3a通过[3 + 2]偶极环加成与t BuNCO反应,得到C 2 OPSi-杂环[(C 6 F 5)3 BHC] CSi(Me 2)P(Ph 2)OC(N t Bu)(10)。此外,4与t BuNCO反应,然后与H 2 O反应,得到(E)-(F 5 C 6)3 BHCC [P(4- t BuC 6 H 4)2 C(O)NH t Bu] [Si(Me 2)OH(NC 5 H 5)](11)通过C 2 OPSi杂环中间体,然后在C 2 OPSi环开口下进行H 2 O分解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号