当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Novel Aryloxyethylamine Derivatives and Evaluation of Their in Vitro and in Vivo Neuroprotective Activities
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2020-09-01 , DOI: 10.1002/cbdv.202000431 Yan Zhong 1 , Yarong Gao 2 , Yi Xu 2 , Changyong Qi 3 , Bin Wu 2
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2020-09-01 , DOI: 10.1002/cbdv.202000431 Yan Zhong 1 , Yarong Gao 2 , Yi Xu 2 , Changyong Qi 3 , Bin Wu 2
Affiliation
|
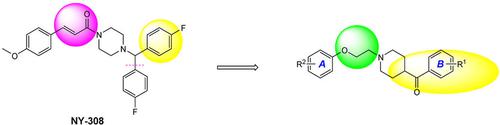
A series of aryloxyethylamine derivatives were designed, synthesized and evaluated for their biological activity. Their structures were confirmed by 1H‐NMR, 13C‐NMR, FT‐IR and HR‐ESI‐MS. The preliminary screening of neuroprotection of compounds in vitro was detected by MTT, and the anti‐ischemic activity in vivo was tested using bilateral common carotid artery occlusion in mice. Most of these compounds showed potential neuroprotective effects against the glutamate‐induced cell death in differentiated rat pheochromocytoma cells (PC12 cells), especially for (4‐fluorophenyl){1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}methanone, {1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}(4‐methoxyphenyl)methanone, (4‐bromophenyl){1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}methanone, {1‐[2‐(4‐chlorophenoxy)ethyl]piperidin‐4‐yl}(4‐chlorophenyl)methanone, (4‐chlorophenyl)(1‐{2‐[(naphthalen‐2‐yl)oxy]ethyl}piperidin‐4‐yl)methanone, (4‐chlorophenyl){1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}methanone and {1‐[2‐(4‐bromophenoxy)ethyl]piperidin‐4‐yl}(4‐chlorophenyl)methanone, which exhibited potent protection of PC12 cells at three doses (0.1, 1.0, 10 μM). Compounds (4‐fluorophenyl){1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}methanone, (4‐fluorophenyl){1‐[2‐(naphthalen‐2‐yloxy)ethyl]piperidin‐4‐yl}methanone, {1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}(4‐methoxyphenyl)methanone and {1‐[2‐(4‐chlorophenoxy)ethyl]piperidin‐4‐yl}(4‐chlorophenyl)methanone possessed the significant prolongation of the survival time of mice subjected to acute cerebral ischemia and decreased the mortality rate at all five doses tested (200, 100, 50, 25, 12.5 mg/kg) and had significant neuroprotective activity. In addition, (4‐fluorophenyl){1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}methanone, {1‐[2‐(4‐methoxyphenoxy)ethyl]piperidin‐4‐yl}(4‐methoxyphenyl)methanone and {1‐[2‐(4‐chlorophenoxy)ethyl]piperidin‐4‐yl}(4‐chlorophenyl)methanone possessed outstanding neuroprotection in vitro and in vivo. These compounds can be used as a promising neuroprotective agents for future development of new anti‐ischemic stroke agents. Basic structure–activity relationships are also presented.
中文翻译:

新型芳氧基乙胺衍生物的合成及其体外和体内神经保护活性的评价
设计、合成了一系列芳氧基乙胺衍生物并评估了它们的生物活性。它们的结构经 1H-NMR、13C-NMR、FT-IR 和 HR-ESI-MS 确证。MTT检测化合物体外神经保护作用的初步筛选,小鼠双侧颈总动脉闭塞实验检测其体内抗缺血活性。这些化合物中的大多数对分化的大鼠嗜铬细胞瘤细胞(PC12 细胞)中谷氨酸诱导的细胞死亡显示出潜在的神经保护作用,特别是对 (4-氟苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮,{1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}(4-甲氧基苯基)甲酮,(4-溴苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4 -基}甲酮,{1-[2-(4-氯苯氧基)乙基]哌啶-4-基}(4-氯苯基)甲酮,(4-氯苯基)(1-{2-[(萘-2-基)氧基]乙基}哌啶-4-基)甲酮,(4-氯苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮和{1-[2-(4-溴苯氧基)乙基]哌啶-4-基}(4-氯苯基)甲酮,在三种剂量(0.1、1.0、10 μM)下表现出对PC12细胞的有效保护)。化合物(4-氟苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮,(4-氟苯基){1-[2-(萘-2-基氧基)乙基]哌啶-4 -基}甲酮,{1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}(4-甲氧基苯基)甲酮和{1-[2-(4-氯苯氧基)乙基]哌啶-4-基} (4-氯苯基)甲酮可显着延长急性脑缺血小鼠的存活时间,并降低所有五种测试剂量(200、100、50、25、12.5 mg/kg)的死亡率,并具有显着的神经保护活性. 此外,(4-氟苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮,{1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}(4-甲氧基苯基)甲酮和{1-[2-(4-氯苯氧基)乙基]哌啶-4-基}(4-氯苯基)甲酮在体外和体内均具有出色的神经保护作用。这些化合物可用作未来开发新型抗缺血性中风药物的有前途的神经保护剂。还介绍了基本的结构-活性关系。这些化合物可用作未来开发新型抗缺血性中风药物的有前途的神经保护剂。还介绍了基本的结构-活性关系。这些化合物可用作未来开发新型抗缺血性中风药物的有前途的神经保护剂。还介绍了基本的结构-活性关系。
更新日期:2020-09-01
中文翻译:

新型芳氧基乙胺衍生物的合成及其体外和体内神经保护活性的评价
设计、合成了一系列芳氧基乙胺衍生物并评估了它们的生物活性。它们的结构经 1H-NMR、13C-NMR、FT-IR 和 HR-ESI-MS 确证。MTT检测化合物体外神经保护作用的初步筛选,小鼠双侧颈总动脉闭塞实验检测其体内抗缺血活性。这些化合物中的大多数对分化的大鼠嗜铬细胞瘤细胞(PC12 细胞)中谷氨酸诱导的细胞死亡显示出潜在的神经保护作用,特别是对 (4-氟苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮,{1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}(4-甲氧基苯基)甲酮,(4-溴苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4 -基}甲酮,{1-[2-(4-氯苯氧基)乙基]哌啶-4-基}(4-氯苯基)甲酮,(4-氯苯基)(1-{2-[(萘-2-基)氧基]乙基}哌啶-4-基)甲酮,(4-氯苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮和{1-[2-(4-溴苯氧基)乙基]哌啶-4-基}(4-氯苯基)甲酮,在三种剂量(0.1、1.0、10 μM)下表现出对PC12细胞的有效保护)。化合物(4-氟苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮,(4-氟苯基){1-[2-(萘-2-基氧基)乙基]哌啶-4 -基}甲酮,{1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}(4-甲氧基苯基)甲酮和{1-[2-(4-氯苯氧基)乙基]哌啶-4-基} (4-氯苯基)甲酮可显着延长急性脑缺血小鼠的存活时间,并降低所有五种测试剂量(200、100、50、25、12.5 mg/kg)的死亡率,并具有显着的神经保护活性. 此外,(4-氟苯基){1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}甲酮,{1-[2-(4-甲氧基苯氧基)乙基]哌啶-4-基}(4-甲氧基苯基)甲酮和{1-[2-(4-氯苯氧基)乙基]哌啶-4-基}(4-氯苯基)甲酮在体外和体内均具有出色的神经保护作用。这些化合物可用作未来开发新型抗缺血性中风药物的有前途的神经保护剂。还介绍了基本的结构-活性关系。这些化合物可用作未来开发新型抗缺血性中风药物的有前途的神经保护剂。还介绍了基本的结构-活性关系。这些化合物可用作未来开发新型抗缺血性中风药物的有前途的神经保护剂。还介绍了基本的结构-活性关系。


























 京公网安备 11010802027423号
京公网安备 11010802027423号