当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polymeric Nanocarriers with Controlled Chain Flexibility Boost mRNA Delivery In Vivo through Enhanced Structural Fastening.
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-06-25 , DOI: 10.1002/adhm.202000538 Takuya Miyazaki 1, 2 , Satoshi Uchida 1 , Satoru Nagatoishi 3 , Kyoko Koji 1 , Taehun Hong 1 , Shigeto Fukushima 4 , Kouhei Tsumoto 1, 3 , Kazuhiko Ishihara 1 , Kazunori Kataoka 4, 5 , Horacio Cabral 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-06-25 , DOI: 10.1002/adhm.202000538 Takuya Miyazaki 1, 2 , Satoshi Uchida 1 , Satoru Nagatoishi 3 , Kyoko Koji 1 , Taehun Hong 1 , Shigeto Fukushima 4 , Kouhei Tsumoto 1, 3 , Kazuhiko Ishihara 1 , Kazunori Kataoka 4, 5 , Horacio Cabral 1
Affiliation
|
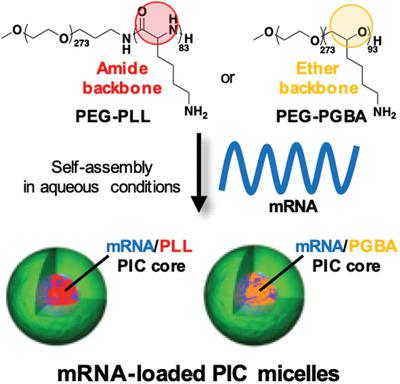
Messenger RNA (mRNA) shows high therapeutic potential, though effective delivery systems are still needed for boosting its application. Nanocarriers loading mRNA via polyion complexation with block catiomers into core‐shell micellar structures are promising systems for enhancing mRNA delivery. Engineering the interaction between mRNA and catiomers through polymer design can promote the development of mRNA‐loaded micelles (mRNA/m) with increased delivery efficiency. Particularly, the polycation chain rigidity may critically affect the mRNA‐catiomer interplay to yield potent nanocarriers, yet its effect remains unknown. Herein, the influence of polycation stiffness on the performance of mRNA/m by developing block complementary catiomers having polycation segments with different flexibility, that is, poly(ethylene glycol)‐poly(glycidylbutylamine) (PEG‐PGBA) and PEG‐poly(L‐lysine) (PEG‐PLL) is studied. PEG‐PGBA allows more than 50‐fold stronger binding to mRNA than the relatively more rigid PEG‐PLL, resulting in mRNA/m with enhanced protection against enzymatic attack and polyanions. mRNA/m from PEG‐PGBA significantly enhances mRNA in vivo bioavailability and increased protein translation, indicating the importance of controlling polycation flexibility for forming stable polyion complexes with mRNA toward improved delivery.
中文翻译:

具有受控链柔性的聚合物纳米载体可通过增强的结构固定来促进mRNA的体内递送。
Messenger RNA(mRNA)具有很高的治疗潜力,尽管仍然需要有效的递送系统来促进其应用。纳米载体通过聚离子与嵌段介聚体的复合作用将mRNA加载到核-壳胶束结构中,是增强mRNA传递的有希望的系统。通过聚合物设计来工程化mRNA和阳离子异构体之间的相互作用,可以提高载有mRNA的胶束(mRNA / m)的发育,并提高递送效率。特别是,聚阳离子链的刚性可能会严重影响mRNA-Casemer相互作用以产生有效的纳米载体,但其作用仍然未知。在本文中,通过开发具有不同柔韧性的聚阳离子链段的嵌段互补阳离子聚合物,即聚阳离子刚度对mRNA / m性能的影响,研究了聚(乙二醇)-聚(缩水甘油基丁胺)(PEG-PGBA)和PEG-聚(L-赖氨酸)(PEG-PLL)。与相对较刚性的PEG-PLL相比,PEG-PGBA可以使与mRNA的结合强度强50倍以上,从而产生mRNA / m值,并增强了对酶攻击和聚阴离子的保护。PEG-PGBA的mRNA / m显着提高了mRNA在体内的生物利用度并增加了蛋白质翻译,这表明控制聚阳离子柔性对于与mRNA形成稳定的聚离子复合物以改善递送的重要性。
更新日期:2020-08-19
中文翻译:

具有受控链柔性的聚合物纳米载体可通过增强的结构固定来促进mRNA的体内递送。
Messenger RNA(mRNA)具有很高的治疗潜力,尽管仍然需要有效的递送系统来促进其应用。纳米载体通过聚离子与嵌段介聚体的复合作用将mRNA加载到核-壳胶束结构中,是增强mRNA传递的有希望的系统。通过聚合物设计来工程化mRNA和阳离子异构体之间的相互作用,可以提高载有mRNA的胶束(mRNA / m)的发育,并提高递送效率。特别是,聚阳离子链的刚性可能会严重影响mRNA-Casemer相互作用以产生有效的纳米载体,但其作用仍然未知。在本文中,通过开发具有不同柔韧性的聚阳离子链段的嵌段互补阳离子聚合物,即聚阳离子刚度对mRNA / m性能的影响,研究了聚(乙二醇)-聚(缩水甘油基丁胺)(PEG-PGBA)和PEG-聚(L-赖氨酸)(PEG-PLL)。与相对较刚性的PEG-PLL相比,PEG-PGBA可以使与mRNA的结合强度强50倍以上,从而产生mRNA / m值,并增强了对酶攻击和聚阴离子的保护。PEG-PGBA的mRNA / m显着提高了mRNA在体内的生物利用度并增加了蛋白质翻译,这表明控制聚阳离子柔性对于与mRNA形成稳定的聚离子复合物以改善递送的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号