当前位置:
X-MOL 学术
›
Mater. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The role of substrate materials on stabilization of CdO, 2CdO·CdSO4 and 2CdS·2CdO·CdSO4 from CdS powder film annealed in air
Materials Chemistry and Physics ( IF 4.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.matchemphys.2020.123251 Davide Casotti , Andrea Gaiardo , Matteo Valt , Barbara Fabbri , Giuseppe Cruciani , Vincenzo Guidi
Materials Chemistry and Physics ( IF 4.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.matchemphys.2020.123251 Davide Casotti , Andrea Gaiardo , Matteo Valt , Barbara Fabbri , Giuseppe Cruciani , Vincenzo Guidi
|
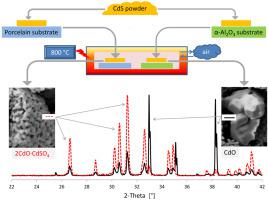
Abstract The relative stability of CdS and CdO with respect of substrates has been widely studied up to 500 °C, the upper temperature in many applications. The physical-chemical properties of the deposited films are often revealed to be influenced by deposition substrates. In this paper, using XRD, SEM and EDX methods, we have studied the oxidation/desulphurization of CdS to CdO and the intermediate stabilized materials therein (i.e. 2CdO·CdSO4 and 2CdS·2CdO·CdSO4), by using CdS films deposited on different substrates (i.e. nickel, Si-polycrystalline, glaze porcelain and α-Al2O3) which were annealed in air up to 800 °C. At the annealing temperatures used in this study, different phases were found to be stabilized on different substrates. The relative stability of CdO and 2CdO·CdSO4 phases is greater on α-Al2O3 than on glazed porcelain substrate. A mixed phase of CdO and 2CdO·CdSO4 was obtained at the annealing temperature of 800 °C if films are deposited on Ni and polycrystalline silica. The amount of the oxidized CdS (i.e. CdO and 2CdO·CdSO4) obtained by mixing CdS and Al2O3 powders at different Al2O3/CdS ratios and annealed up to 800 °C, were also investigated. Decreasing the Al2O3/CdS ratio, the interactions between Al2O3 and CdS particles are reduced, consequently the CdO stabilization is strongly retarded in favour of 2CdO·CdSO4, while reverse results was proven for high values of Al2O3/CdS ratio. The results show the role of the interface area between Al2O3 and Cd-materials for stabilization of CdO. A low interface area was also obtained by filling an α-Al2O3 crucible with CdS powder. After annealing, the CdO stabilization was strongly reduced, in line with the low Al2O3/CdS ratio sample. Spreading the CdS powder on the α-Al2O3 substrate, instead of drop coating deposition method, showed a remarkable CdO stabilization in line with the results obtained with drop coating method. To account for the role of the interface area a model is proposed (Stabilization model), based on surface energy, as responsible to phase stabilization to explain the nucleation – stabilization of the CdO and 2CdS·2CdO·CdSO4. The morphologies of the CdO and 2CdS·2CdO·CdSO4 resemble hexagonal-prism shapes and compact chips, respectively, while 2CdO·CdSO4 phases has a sponge-like appearance.
中文翻译:

衬底材料对空气中退火的 CdS 粉末薄膜中 CdO、2CdO·CdSO4 和 2CdS·2CdO·CdSO4 的稳定作用
摘要 CdS 和 CdO 相对于衬底的相对稳定性已被广泛研究,直到 500 °C,这是许多应用中的上限温度。沉积薄膜的物理化学性质通常会受到沉积基材的影响。在本文中,我们使用 XRD、SEM 和 EDX 方法,通过使用沉积在不同基材上的 CdS 薄膜,研究了 CdS 氧化/脱硫为 CdO 及其中间稳定材料(即 2CdO·CdSO4 和 2CdS·2CdO·CdSO4) (即镍、多晶硅、釉瓷和 α-Al2O3)在空气中退火至 800 °C。在本研究中使用的退火温度下,发现不同的相在不同的基材上稳定。CdO 和 2CdO·CdSO4 相在 α-Al2O3 上的相对稳定性比在釉瓷基板上大。如果薄膜沉积在 Ni 和多晶二氧化硅上,则在 800 °C 的退火温度下获得 CdO 和 2CdO·CdSO4 的混合相。还研究了通过以不同的 Al2O3/CdS 比率混合 CdS 和 Al2O3 粉末并在高达 800°C 下退火获得的氧化 CdS(即 CdO 和 2CdO·CdSO4)的量。降低 Al2O3/CdS 比值,Al2O3 和 CdS 颗粒之间的相互作用减少,因此 CdO 稳定化受到强烈阻碍,有利于 2CdO·CdSO4,而相反的结果证明了高值 Al2O3/CdS 比值。结果显示了 Al2O3 和 Cd 材料之间的界面区域对 CdO 的稳定化的作用。通过用 CdS 粉末填充 α-Al2O3 坩埚也获得了低界面面积。退火后,CdO 的稳定性大大降低,符合低 Al2O3/CdS 比率样品。将 CdS 粉末涂抹在 α-Al2O3 基材上,而不是滴涂沉积方法,显示出与滴涂法获得的结果一致的显着 CdO 稳定性。为了解释界面区域的作用,提出了一个基于表面能的模型(稳定模型),负责相稳定以解释成核 - 稳定化 CdO 和 2CdS·2CdO·CdSO4。CdO和2CdS·2CdO·CdSO4的形态分别类似于六棱柱形和致密的碎片,而2CdO·CdSO4相具有海绵状外观。为了解释界面区域的作用,提出了一个基于表面能的模型(稳定模型),负责相稳定以解释成核 - 稳定化 CdO 和 2CdS·2CdO·CdSO4。CdO和2CdS·2CdO·CdSO4的形态分别类似于六棱柱形和致密的碎片,而2CdO·CdSO4相具有海绵状外观。为了解释界面区域的作用,提出了一个基于表面能的模型(稳定模型),负责相稳定以解释成核 - 稳定化 CdO 和 2CdS·2CdO·CdSO4。CdO和2CdS·2CdO·CdSO4的形态分别类似于六棱柱形和致密的碎片,而2CdO·CdSO4相具有海绵状外观。
更新日期:2021-01-01
中文翻译:

衬底材料对空气中退火的 CdS 粉末薄膜中 CdO、2CdO·CdSO4 和 2CdS·2CdO·CdSO4 的稳定作用
摘要 CdS 和 CdO 相对于衬底的相对稳定性已被广泛研究,直到 500 °C,这是许多应用中的上限温度。沉积薄膜的物理化学性质通常会受到沉积基材的影响。在本文中,我们使用 XRD、SEM 和 EDX 方法,通过使用沉积在不同基材上的 CdS 薄膜,研究了 CdS 氧化/脱硫为 CdO 及其中间稳定材料(即 2CdO·CdSO4 和 2CdS·2CdO·CdSO4) (即镍、多晶硅、釉瓷和 α-Al2O3)在空气中退火至 800 °C。在本研究中使用的退火温度下,发现不同的相在不同的基材上稳定。CdO 和 2CdO·CdSO4 相在 α-Al2O3 上的相对稳定性比在釉瓷基板上大。如果薄膜沉积在 Ni 和多晶二氧化硅上,则在 800 °C 的退火温度下获得 CdO 和 2CdO·CdSO4 的混合相。还研究了通过以不同的 Al2O3/CdS 比率混合 CdS 和 Al2O3 粉末并在高达 800°C 下退火获得的氧化 CdS(即 CdO 和 2CdO·CdSO4)的量。降低 Al2O3/CdS 比值,Al2O3 和 CdS 颗粒之间的相互作用减少,因此 CdO 稳定化受到强烈阻碍,有利于 2CdO·CdSO4,而相反的结果证明了高值 Al2O3/CdS 比值。结果显示了 Al2O3 和 Cd 材料之间的界面区域对 CdO 的稳定化的作用。通过用 CdS 粉末填充 α-Al2O3 坩埚也获得了低界面面积。退火后,CdO 的稳定性大大降低,符合低 Al2O3/CdS 比率样品。将 CdS 粉末涂抹在 α-Al2O3 基材上,而不是滴涂沉积方法,显示出与滴涂法获得的结果一致的显着 CdO 稳定性。为了解释界面区域的作用,提出了一个基于表面能的模型(稳定模型),负责相稳定以解释成核 - 稳定化 CdO 和 2CdS·2CdO·CdSO4。CdO和2CdS·2CdO·CdSO4的形态分别类似于六棱柱形和致密的碎片,而2CdO·CdSO4相具有海绵状外观。为了解释界面区域的作用,提出了一个基于表面能的模型(稳定模型),负责相稳定以解释成核 - 稳定化 CdO 和 2CdS·2CdO·CdSO4。CdO和2CdS·2CdO·CdSO4的形态分别类似于六棱柱形和致密的碎片,而2CdO·CdSO4相具有海绵状外观。为了解释界面区域的作用,提出了一个基于表面能的模型(稳定模型),负责相稳定以解释成核 - 稳定化 CdO 和 2CdS·2CdO·CdSO4。CdO和2CdS·2CdO·CdSO4的形态分别类似于六棱柱形和致密的碎片,而2CdO·CdSO4相具有海绵状外观。


























 京公网安备 11010802027423号
京公网安备 11010802027423号