Archives of Biochemistry and Biophysics ( IF 3.9 ) Pub Date : 2020-06-25 , DOI: 10.1016/j.abb.2020.108446 Naoko Iwaya 1 , Natsuko Goda 2 , Mizuki Matsuzaki 3 , Akihiro Narita 3 , Yoshiki Shigemitsu 4 , Takeshi Tenno 5 , Yoshito Abe 6 , Minako Hoshi 7 , Hidekazu Hiroaki 5
|
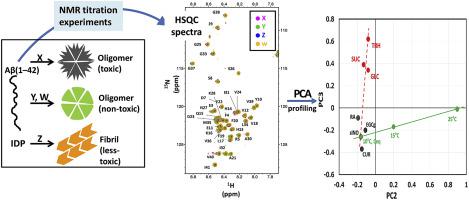
A simple NMR method to analyze the data obtained by NMR titration experiment of amyloid formation inhibitors against uniformly 15N-labeled amyloid-β 1–42 peptide (Aβ(1–42)) was described. By using solution nuclear magnetic resonance (NMR) measurement, the simplest method for monitoring the effects of Aβ fibrilization inhibitors is the NMR chemical shift perturbation (CSP) experiment using 15N-labeled Aβ(1–42). However, the flexible and dynamic nature of Aβ(1–42) monomer may hamper the interpretation of CSP data. Here we introduced principal component analysis (PCA) for visualizing and analyzing NMR data of Aβ(1–42) in the presence of amyloid inhibitors including high concentration osmolytes. We measured 1H–15N 2D spectra of Aβ(1–42) at various temperatures as well as of Aβ(1–42) with several inhibitors, and subjected all the data to PCA (PCA-HSQC). The PCA diagram succeeded in differentiating the various amyloid inhibitors, including epigallocatechin gallate (EGCg), rosmarinic acid (RA) and curcumin (CUR) from high concentration osmolytes. We hypothesized that the CSPs reflected the conformational equilibrium of intrinsically disordered Aβ(1–42) induced by weak inhibitor binding rather than the specific molecular interactions.
中文翻译:

来自15N标记的淀粉样β(1-42)肽与渗透液和酚类化合物的NMR滴定实验的数据的主成分分析。
描述了一种简单的NMR方法,用于分析通过NMR滴定实验对15种N标记的N-淀粉样β-1-42肽(Aβ(1-42))进行均匀抑制的数据。通过使用溶液核磁共振(NMR)测量,监测Aβ纤化抑制剂作用的最简单方法是使用15种N标记的Aβ(1-42)进行的NMR化学位移扰动(CSP)实验。但是,Aβ(1-42)单体的柔性和动态性质可能会妨碍CSP数据的解释。在这里,我们介绍了主成分分析(PCA),用于在淀粉样蛋白抑制剂(包括高浓度渗透剂)存在下可视化和分析Aβ(1-42)的NMR数据。我们测量了1 H– 15Aβ(1-42)在各种温度下以及带有几种抑制剂的Aβ(1-42)的N 2D光谱,并将所有数据进行PCA(PCA-HSQC)处理。PCA图成功地区分了各种淀粉样蛋白抑制剂,包括表没食子儿茶素没食子酸酯(EGCg),迷迭香酸(RA)和姜黄素(CUR)与高浓度渗透压剂。我们假设CSP反映了弱抑制剂结合而不是特定的分子相互作用引起的内在无序的Aβ(1-42)的构象平衡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号