当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Homochiral Covalent Organic Framework for Catalytic Asymmetric Synthesis of Drug Intermediate
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c04722 Hui-Chao Ma 1 , Gong-Jun Chen 1 , Fang Huang 1 , Yu-Bin Dong 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c04722 Hui-Chao Ma 1 , Gong-Jun Chen 1 , Fang Huang 1 , Yu-Bin Dong 1
Affiliation
|
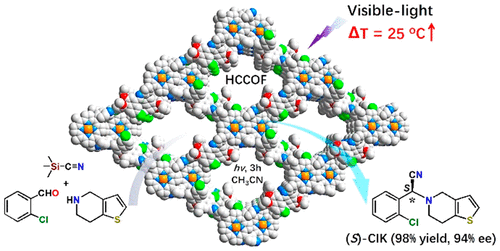
(S)-2-(2-Chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)acetonitrile ((S)-CIK) is a key intermediate to (S)-clopidogrel which is one of the most salable worldwide antiplatelet and antithrombotic drugs. We show herein a facile meth-od for direct synthesis of (S)-CIK via one-pot in situ Strecker reaction using homochiral covalent framework catalyst in a het-erogeneous way. The asymmetric synthesis herein involves a photothermal conversion triggered thermally-driven reaction which affords (S)-CIK in 98% yield with 94% enantiomeric excess under visible-light irradiation. Furthermore, the above approach is readily extended to a gram-scale production on a fixed-bed continuous-flow model reactor. The potential utility of this strategy is highlighted by the preparation of many more other types of chiral drugs and drug intermediates in a green and facile way.
中文翻译:

用于催化不对称合成药物中间体的同手性共价有机骨架
(S)-2-(2-氯苯基)-2-(6,7-二氢噻吩并[3,2-c]吡啶-5(4H)-基)乙腈((S)-CIK)是( S)-氯吡格雷,它是全球最畅销的抗血小板和抗血栓药物之一。我们在此展示了一种使用同手性共价骨架催化剂以异相方式通过一锅原位 Strecker 反应直接合成 (S)-CIK 的简便方法。本文的不对称合成涉及光热转化引发的热驱动反应,其在可见光照射下以 98% 的产率提供 (S)-CIK,对映体过量为 94%。此外,上述方法很容易扩展到固定床连续流动模型反应器上的克级生产。
更新日期:2020-06-23
中文翻译:

用于催化不对称合成药物中间体的同手性共价有机骨架
(S)-2-(2-氯苯基)-2-(6,7-二氢噻吩并[3,2-c]吡啶-5(4H)-基)乙腈((S)-CIK)是( S)-氯吡格雷,它是全球最畅销的抗血小板和抗血栓药物之一。我们在此展示了一种使用同手性共价骨架催化剂以异相方式通过一锅原位 Strecker 反应直接合成 (S)-CIK 的简便方法。本文的不对称合成涉及光热转化引发的热驱动反应,其在可见光照射下以 98% 的产率提供 (S)-CIK,对映体过量为 94%。此外,上述方法很容易扩展到固定床连续流动模型反应器上的克级生产。



























 京公网安备 11010802027423号
京公网安备 11010802027423号