当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and Evaluation of a Detailed Mechanism for Gas-Phase Atmospheric Reactions of Furans
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-06-24 , DOI: 10.1021/acsearthspacechem.0c00058 Jia Jiang 1, 2 , William P. L. Carter 2 , David R. Cocker 1, 2 , Kelley C. Barsanti 1, 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-06-24 , DOI: 10.1021/acsearthspacechem.0c00058 Jia Jiang 1, 2 , William P. L. Carter 2 , David R. Cocker 1, 2 , Kelley C. Barsanti 1, 2
Affiliation
|
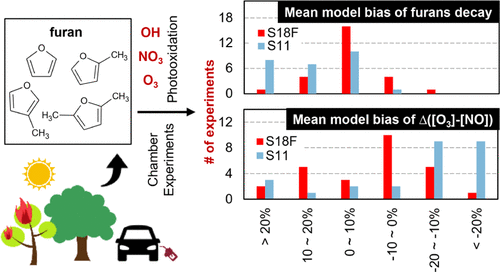
Furan and its alkyl derivatives (furans) are emitted to the atmosphere from multiple sources and can exist in sufficient quantities to affect atmospheric oxidant levels and secondary pollutant formation. Such compounds and their chemical transformations are generally oversimplified in gas-phase chemical mechanisms used for air quality predictions and atmospheric modeling studies. Furans are typically lumped as reactive aromatics, which largely underpredicts their oxidation rates. This work presents a detailed gas-phase mechanism for furans and their major oxidation products. The reactions and rate constants were derived using published data and the Statewide Air Pollution Research Center (SAPRC) mechanism generation system (MechGen). The detailed furans mechanism was implemented in the SAPRC-18 base mechanism to enable evaluation against environmental chamber experiments. A reduced version of the mechanism was developed that maintains consistency and compatibility with the SAPRC-07/-11 base mechanisms. Relative to the lumped SAPRC-11 mechanism, the model skill was improved in predicting furans consumption (21 of 26 experiments) and Δ([O3] – [NO]). For the latter, mean model bias was reduced to ±10% for 13 experiments (4 for SAPRC-11) and was >±20% for only 3 experiments (11 for SAPRC-11). Sensitivity simulations were performed to evaluate the relative importance of hydroxyl radical (OH)-, nitrate radical (NO3)-, and ozone (O3)-initiated photo-oxidation. While OH-initiated reactions are the major sink for furans, reactions with NO3 and O3 become non-negligible or even of equal importance under dark or high O3 conditions, such as may be expected during biomass burning events. The detailed furans mechanism was developed with no tuning to fit the experimental data used for evaluation and to facilitate the broad application of the mechanism for atmospheric and air quality modeling.
中文翻译:

呋喃气相大气反应详细机理的开发与评价
呋喃及其烷基衍生物(呋喃)从多种来源排放到大气中,其存在量足以影响大气中的氧化剂水平和二次污染物的形成。在用于空气质量预测和大气模拟研究的气相化学机理中,此类化合物及其化学转化通常被过分简化。呋喃通常被混为反应性芳族化合物,这大大低估了它们的氧化速率。这项工作提出了呋喃及其主要氧化产物的详细气相机理。反应和速率常数是使用已发布的数据和全州空气污染研究中心(SAPRC)机制生成系统(MechGen)得出的。在SAPRC-18基本机制中实施了详细的呋喃机制,以能够针对环境室实验进行评估。开发了该机制的简化版本,以保持与SAPRC-07 / -11基本机制的一致性和兼容性。相对于集总SAPRC-11机制,该模型在预测呋喃消耗量(26个实验中的21个)和Δ([O3 ] – [否])。对于后者,平均模型偏差对于13个实验(对于SAPRC-11为4个)降低到±10%,对于仅3个实验(对于SAPRC-11,为11个)>> 20%。进行了敏感性模拟,以评估羟基自由基(OH)-,硝酸根自由基(NO 3)-和臭氧(O 3)引发的光氧化的相对重要性。尽管OH引发的反应是呋喃的主要吸收源,但在黑暗或高O 3下,与NO 3和O 3的反应变得不可忽略甚至同等重要。条件,例如在生物质燃烧事件期间可能会发生的情况。开发了详细的呋喃机制,没有进行任何调整,以适应用于评估的实验数据并促进该机制在大气和空气质量建模中的广泛应用。
更新日期:2020-08-20
中文翻译:

呋喃气相大气反应详细机理的开发与评价
呋喃及其烷基衍生物(呋喃)从多种来源排放到大气中,其存在量足以影响大气中的氧化剂水平和二次污染物的形成。在用于空气质量预测和大气模拟研究的气相化学机理中,此类化合物及其化学转化通常被过分简化。呋喃通常被混为反应性芳族化合物,这大大低估了它们的氧化速率。这项工作提出了呋喃及其主要氧化产物的详细气相机理。反应和速率常数是使用已发布的数据和全州空气污染研究中心(SAPRC)机制生成系统(MechGen)得出的。在SAPRC-18基本机制中实施了详细的呋喃机制,以能够针对环境室实验进行评估。开发了该机制的简化版本,以保持与SAPRC-07 / -11基本机制的一致性和兼容性。相对于集总SAPRC-11机制,该模型在预测呋喃消耗量(26个实验中的21个)和Δ([O3 ] – [否])。对于后者,平均模型偏差对于13个实验(对于SAPRC-11为4个)降低到±10%,对于仅3个实验(对于SAPRC-11,为11个)>> 20%。进行了敏感性模拟,以评估羟基自由基(OH)-,硝酸根自由基(NO 3)-和臭氧(O 3)引发的光氧化的相对重要性。尽管OH引发的反应是呋喃的主要吸收源,但在黑暗或高O 3下,与NO 3和O 3的反应变得不可忽略甚至同等重要。条件,例如在生物质燃烧事件期间可能会发生的情况。开发了详细的呋喃机制,没有进行任何调整,以适应用于评估的实验数据并促进该机制在大气和空气质量建模中的广泛应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号