Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
USP28 and USP25 are downregulated by Vismodegib in vitro and in colorectal cancer cell lines
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-06-23 , DOI: 10.1111/febs.15461 Hui Wang 1, 2 , Qian Meng 3 , Yiluan Ding 1 , Muya Xiong 2, 3 , Mengying Zhu 1, 2 , Yuanyuan Yang 1, 2 , Haixia Su 2, 3 , Lei Gu 2, 3 , Yechun Xu 2, 3 , Li Shi 1 , Hu Zhou 2, 3 , Naixia Zhang 1, 2
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-06-23 , DOI: 10.1111/febs.15461 Hui Wang 1, 2 , Qian Meng 3 , Yiluan Ding 1 , Muya Xiong 2, 3 , Mengying Zhu 1, 2 , Yuanyuan Yang 1, 2 , Haixia Su 2, 3 , Lei Gu 2, 3 , Yechun Xu 2, 3 , Li Shi 1 , Hu Zhou 2, 3 , Naixia Zhang 1, 2
Affiliation
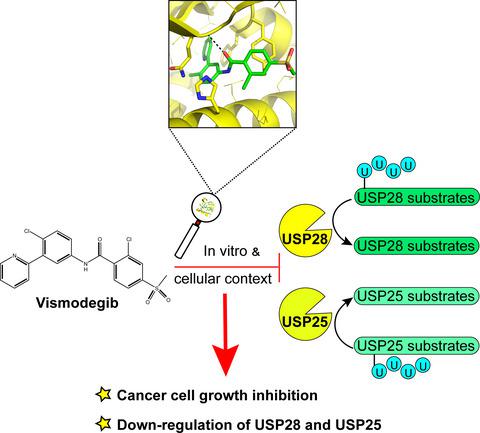
|
Deubiquitinase USP28 plays a crucial role in tumorigenesis by enhancing the stabilities of multiple cancer‐related proteins including c‐Myc, Notch1, and LSD1, and has become an attractive target for anticancer drug development. However, to date, only a few of USP28‐targeted active compounds have been developed, and the active compound‐binding pocket in USP28 has not been experimentally revealed yet. In this study, bioassay‐based high‐throughput screening was applied to discover USP28‐targeted inhibitors from the commercially available drug library. Vismodegib, an inhibitor of Hedgehog signaling pathway and FDA‐approved drug for the treatment of basal cell carcinoma, was found to exhibit inhibition activity against USP28 (IC50: 4.41 ± 1.08 μm). Multiple biophysical and biochemical techniques including NMR, ITC, thermal shift assay, HDX‐MS, and site‐directed mutagenesis analysis were then used to characterize the interaction between Vismodegib and USP28. The binding pocket in USP28 for Vismodegib, which is mainly composed of two helical structures spanning D255‐N278 and N286‐Y293, was revealed. According to the possible binding pose generated by HDX‐MS data‐defined molecular docking, the binding cavity occupied by Vismodegib in USP28 aligns well with one of the reported‐binding pockets in USP7 for its inhibitors. Furthermore, cellular assays were conducted to confirm that Vismodegib could interact with the evolutionarily related deubiquitinases USP28 and USP25 and downregulate the levels of the two enzymes' substrate proteins c‐Myc, Notch1, and Tankyrase‐1/2.
中文翻译:

Vismodegib在体外和结直肠癌细胞系中下调USP28和USP25
去泛素化酶USP28通过增强多种与癌症相关的蛋白质(包括c-Myc,Notch1和LSD1)的稳定性在肿瘤发生中起关键作用,并已成为抗癌药物开发的有吸引力的靶标。但是,迄今为止,只开发了少数针对USP28的活性化合物,而且尚未通过实验揭示出USP28中的活性化合物结合口袋。在这项研究中,基于生物测定的高通量筛选被用于从市售药物库中发现针对USP28的抑制剂。Vismodegib,基底细胞癌的治疗中的Hedgehog信号传导途径和FDA批准的药物的抑制剂,被发现表现出抑制活性对USP28(IC 50:4.41±1.08μ米)。然后使用多种生物物理和生化技术,包括NMR,ITC,热位移分析,HDX-MS和定点诱变分析来表征Vismodegib和USP28之间的相互作用。揭示了USP28中对Vismodegib的结合口袋,该结合口袋主要由跨越D255-N278和N286-Y293的两个螺旋结构组成。根据HDX-MS数据定义的分子对接产生的可能的结合姿势,Vismodegib在USP28中占据的结合腔与USP7中报道的其抑制剂的结合口袋之一很好地对齐。此外,进行了细胞测定以确认Vismodegib可以与进化相关的去泛素酶USP28和USP25相互作用,并下调两种酶的底物蛋白c-Myc,Notch1和Tankyrase-1 / 2的水平。
更新日期:2020-06-23
中文翻译:

Vismodegib在体外和结直肠癌细胞系中下调USP28和USP25
去泛素化酶USP28通过增强多种与癌症相关的蛋白质(包括c-Myc,Notch1和LSD1)的稳定性在肿瘤发生中起关键作用,并已成为抗癌药物开发的有吸引力的靶标。但是,迄今为止,只开发了少数针对USP28的活性化合物,而且尚未通过实验揭示出USP28中的活性化合物结合口袋。在这项研究中,基于生物测定的高通量筛选被用于从市售药物库中发现针对USP28的抑制剂。Vismodegib,基底细胞癌的治疗中的Hedgehog信号传导途径和FDA批准的药物的抑制剂,被发现表现出抑制活性对USP28(IC 50:4.41±1.08μ米)。然后使用多种生物物理和生化技术,包括NMR,ITC,热位移分析,HDX-MS和定点诱变分析来表征Vismodegib和USP28之间的相互作用。揭示了USP28中对Vismodegib的结合口袋,该结合口袋主要由跨越D255-N278和N286-Y293的两个螺旋结构组成。根据HDX-MS数据定义的分子对接产生的可能的结合姿势,Vismodegib在USP28中占据的结合腔与USP7中报道的其抑制剂的结合口袋之一很好地对齐。此外,进行了细胞测定以确认Vismodegib可以与进化相关的去泛素酶USP28和USP25相互作用,并下调两种酶的底物蛋白c-Myc,Notch1和Tankyrase-1 / 2的水平。


























 京公网安备 11010802027423号
京公网安备 11010802027423号