当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral Self-Sorting Effects in the Self-Assembly of Metallosupramolecular Aggregates Comprising Ligands Derived from Tröger's Base.
ChemPlusChem ( IF 3.4 ) Pub Date : 2020-06-24 , DOI: 10.1002/cplu.202000387 Andreas Jarzebski 1 , Gregor Schnakenburg 2 , Arne Lützen 1
ChemPlusChem ( IF 3.4 ) Pub Date : 2020-06-24 , DOI: 10.1002/cplu.202000387 Andreas Jarzebski 1 , Gregor Schnakenburg 2 , Arne Lützen 1
Affiliation
|
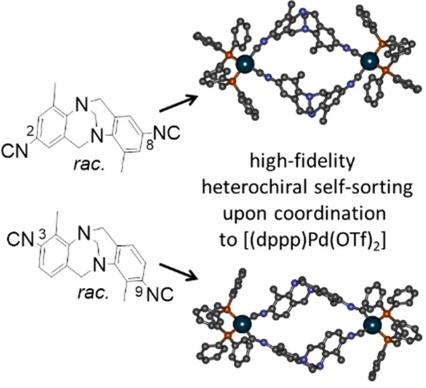
Five ligands with either nitrile or isonitrile metal binding motifs have been synthesized based on the 2,8‐ or 3,9‐disubstituted Tröger's base scaffold, respectively. These ligands self‐assemble into dinuclear cyclic metallosupramolecular aggregates upon coordination to [(dppp)Pd(OTf)2] in a highly diastereoselective manner, by heterochiral self‐sorting in a chiral self‐discriminating manner as shown by ESI mass spectrometry, NMR spectroscopy, and single crystal XRD analysis. This observation is in contrast to earlier studies with ligands derived from Tröger's base that have larger metal binding motifs and bis(nitrile) and bis(isonitrile) ligands based on other rigid dissymmetric cores such as [2.2]paracyclophanes. Thus, the combination of these slim metal binding motifs with the rigid v‐shaped 2,8‐ or 3,9‐disubstituted Tröger's base scaffolds seems to be especially well preorganized to ensure high‐fidelity social self‐sorting behavior.
中文翻译:

包含来自Tröger碱的配体的金属超分子聚集体的自组装中的手性自分选效应。
分别基于2,8-或3,9-二取代的Tröger碱基支架合成了五个具有腈或异腈金属结合基序的配体。这些配体与[(dppp)Pd(OTf)2配位后自组装成双核环状金属超分子聚集体]通过ESI质谱,NMR光谱和单晶XRD分析显示,以手性自甄别方式进行异手性自分选,从而具有非对映选择性。该观察结果与早期的研究相对照,该研究使用的是来自Tröger碱的配体,该配体具有较大的金属结合基序,并且基于其他刚性不对称核(例如[2.2]对环环烷)的双(腈)和双(异腈)配体。因此,这些细长的金属结合基序与刚性的v型2,8-或3,9-二取代Tröger的基础支架相结合似乎可以很好地进行预组织,以确保高保真的社会自我分类行为。
更新日期:2020-07-09
中文翻译:

包含来自Tröger碱的配体的金属超分子聚集体的自组装中的手性自分选效应。
分别基于2,8-或3,9-二取代的Tröger碱基支架合成了五个具有腈或异腈金属结合基序的配体。这些配体与[(dppp)Pd(OTf)2配位后自组装成双核环状金属超分子聚集体]通过ESI质谱,NMR光谱和单晶XRD分析显示,以手性自甄别方式进行异手性自分选,从而具有非对映选择性。该观察结果与早期的研究相对照,该研究使用的是来自Tröger碱的配体,该配体具有较大的金属结合基序,并且基于其他刚性不对称核(例如[2.2]对环环烷)的双(腈)和双(异腈)配体。因此,这些细长的金属结合基序与刚性的v型2,8-或3,9-二取代Tröger的基础支架相结合似乎可以很好地进行预组织,以确保高保真的社会自我分类行为。



























 京公网安备 11010802027423号
京公网安备 11010802027423号