Water Research ( IF 12.8 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.watres.2020.116056 Qingqing Kong 1 , Xin Lei 1 , Xinran Zhang 1 , Shuangshuang Cheng 1 , Chao Xu 2 , Bin Yang 2 , Xin Yang 1
|
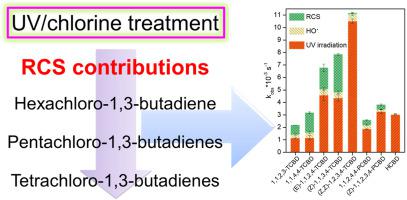
Polychoro-1,3-butadienes (CBDs) were widely found in aqueous environment and resistant to conventional water treatment. In this study, the abatement of CBDs during UV/chlorine treatment was investigated. In comparison to UV irradiation alone, free chlorine addition brought benefits for the reduction of tetra-CBDs (TCBDs), but to lesser extent for penta-CBDs (PCBDs), and virtually no benefit for hexa-CBD (HCBD). At a UV dose of 128 mJ cm−2 and a chlorine dose of 10 mg L−1, about 71.7–97.8% CBDs were degraded by UV/chlorine treatment within 10 min. UV irradiation contributed 32.8%–97.6%, HO• contributed 2.6%–14.4%, and reactive chlorine species (RCS) contributed less than 0.5%–42.3% to CBDs degradation. The percentages of RCS contribution generally followed the order of TCBDs (except (Z,Z)-1,2,3,4-TCBD) > PCBDs > HCBD. The chlorine oxide radical (ClO•) was the dominant RCS contributing to the degradation of CBDs. The second-order reaction rate constants of ClO• with CBDs () were at ∼ 107 M−1s−1 except (Z,Z)-1,2,3,4-TCBD and HCBD (˂ 106 M−1s−1). generally decreased with increasing numbers of chlorine atoms and was also affected by the positions of chlorine atoms in CBDs. A distinct reaction pathway of ClO•, with (Z)-1,1,2,3,4-PCBD as a representative CBD, was proposed. Photoisomers of CBDs from Z or E configuration were observed at lower concentrations in UV/chlorine treatment than under UV irradiation alone due to the radical-involved oxidation, but more organic acids including oxalic acid were observed. In a natural water sample, UV/chlorine treatment also exhibited a good performance in abatement of TCBDs and PCBDs, but not in abatement of HCBD.
中文翻译:

在紫外线/氯气处理中,氧化氯自由基(ClO•)在降解1,-3-丁二烯中的作用:动力学和机理。
在水性环境中广泛发现多氯-1,3-丁二烯(CBD),并且耐常规水处理。在这项研究中,研究了紫外线/氯气处理过程中CBD的减少。与单独的紫外线照射相比,添加游离氯为减少四-CBD(TCBD)带来了好处,但对五-CBD(PCBD)的影响却较小,而对六-CBD(HCBD)几乎没有好处。在UV剂量为128 mJ cm -2和氯剂量为10 mg L -1的情况下,在10分钟内通过UV /氯处理降解了约71.7–97.8%的CBD。紫外线照射占32.8%–97.6%,HO •贡献了2.6%–14.4%,而活性氯物种(RCS)对CBD的降解贡献不到0.5%–42.3%。RCS贡献的百分比通常遵循TCBD的顺序((Z,Z)-1,2,3,4-TCBD除外)> PCBD> HCBD。氧化氯自由基(ClO •)是导致CBD降解的主要RCS。ClO •与CBDs的二阶反应速率常数()为〜10 7 M -1 s -1,(Z,Z)-1、2、3、4-TCBD和HCBD为(10 6 M -1 s -1)。通常随着氯原子数量的增加而减少,并且还受CBD中氯原子位置的影响。提出了以(Z)-1,1,2,3,4-PCBD为代表的CBD的ClO •的独特反应途径。由于自由基参与的氧化作用,在Z / E构型的CBD的光异构体在UV /氯处理中的浓度低于单独在UV照射下的浓度,但观察到更多的有机酸,包括草酸。在天然水样品中,UV /氯处理在减少TCBD和PCBD方面也表现出良好的性能,但在减少HCBD方面却没有。



























 京公网安备 11010802027423号
京公网安备 11010802027423号