Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.jorganchem.2020.121359 Felix Odame , Guillaume Woodcock , Eric C. Hosten , Kevin Lobb , Zenixole R. Tshentu
|
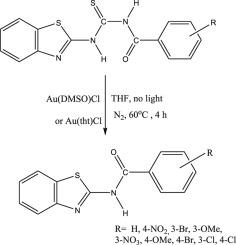
A novel gold(I)-mediated intramolecular transamidation of thiourea derivatives to yield benzamides via dethiocyanation have been achieved by the reaction of 3-(1,3-benzothiazol-2-yl)-1-(benzoyl)thiourea derivatives in the presence of gold(I) precursors. The compounds have been characterized using IR, NMR, GC-MS and microanalysis. The single crystal XRD of 3-(1,3-benzothiazol-2-yl)-1-(3-bromobenzoyl)thiourea (5), 3-(1,3-benzothiazol-2-yl)-1-(3-methoxybenzoyl)thiourea (6), N-(benzothiazol-2-yl)benzamide (10), N-(benzothiazol-2-yl)-3-chlorobenzamide (11), N-(benzothiazol-2-yl)-4-nitrobenzamide (12), N-(benzothiazol-2-yl)-3-bromobenzamide (14) have been discussed. The novel transformation is thought to proceed by a gold(I)-mediated intramolecular transamidation reaction which releases thiocyanate to yield the benzamide. Density functional theory calculations have been used to support the proposed mechanism for this transformation.
中文翻译:

新型金(I)介导的苯甲酰基硫脲衍生物的分子内氨基转移通过脱硫氰化反应形成苯甲酰胺
通过3-(1,3-苯并噻唑-2-基)-1-(苯甲酰基)硫脲衍生物的反应,实现了一种新的金(I)介导的硫脲衍生物分子内酰胺基转移,通过脱硫氰化反应生成苯甲酰胺。金(I)前体。使用IR,NMR,GC-MS和微量分析对化合物进行了表征。3-(1,3-苯并噻唑-2-基)-1-(3-溴苯甲酰基)硫脲(5),3-(1,3-苯并噻唑-2-基)-1-(3-甲氧基苯甲酰基)硫脲(6),N-(苯并噻唑-2-基)苯甲酰胺(10),N-(苯并噻唑-2-基)-3-氯苯甲酰胺(11),N-(苯并噻唑-2-基)-4-硝基苯甲酰胺(12),已经讨论了N-(苯并噻唑-2-基)-3-溴苯甲酰胺(14)。新的转化被认为是通过金(I)介导的分子内氨基转移反应进行的,该反应释放出硫氰酸盐以产生苯甲酰胺。密度泛函理论计算已用于支持此转换的建议机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号