Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.jinorgbio.2020.111157 Alma Salim 1 , Serge Chesnov 2 , Eva Freisinger 1
|
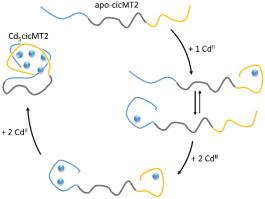
The plant metallothionein 2 protein from Cicer arietinum (cicMT2) is a typical member of the plant MT subfamily p2 that is characterized by an N- and C-terminal cysteine- (Cys-)rich, metal binding sequence connected by a long cysteine-free linker region. cicMT2 coordinates up to five ZnII or CdII ions by its 14 cysteine thiolate groups forming a single metal-thiolate cluster. While MTs from other phyla are considerably well-studied, many details about plant MTs are missing. In this study the metallation pathway of cicMT2 is investigated using mass spectrometry. To evaluate the influence of the linker region as well as the interplay of the two Cys-rich stretches, the full-length cicMT2 protein as well as the individual Cys-rich domains with and without the linker region were analysed. Up to three CdII ions can be coordinated by the eight Cys residues of the N-terminal part and up to two CdII ions by the six Cys residues of the C-terminal sequence. However, no preferential binding to either of the two sequences is observed, which is in-line with the closely similar apparent binding constants of the individual domains obtained from competition reactions with the chelator 1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid. The combination of limited proteolytic digestion, mass spectrometry, dynamic light scattering, size-exclusion chromatography, and 19F NMR spectroscopy enables us to draw conclusions about the overall protein-fold and the cluster formation process.
中文翻译:

植物金属硫蛋白的金属化途径:Ariecerum MT2。
姬松果中的植物金属硫蛋白2蛋白(cicMT2)是植物MT亚科p2的典型成员,其特征在于富含N和C端半胱氨酸(Cys-)的金属结合序列,通过长无半胱氨酸连接接头区域。cicMT2最多可协调五个Zn II或Cd II离子通过其14个半胱氨酸硫醇盐基团形成一个金属硫醇盐簇。尽管对其他门类的MT进行了充分的研究,但缺少有关植物MT的许多细节。在这项研究中,使用质谱法研究了cicMT2的金属化途径。为了评估接头区域的影响以及两个富含Cys的延伸片段的相互作用,分析了全长cicMT2蛋白以及具有和不具有接头区域的单个富含Cys的结构域。N末端部分的八个Cys残基可协调多达三个Cd II离子,而多达两个Cd IIC-末端序列的六个Cys残基形成离子。但是,没有观察到与两个序列中任何一个的优先结合,这与从与螯合剂1,2-双(2-氨基-5-氟苯氧基)的竞争反应中获得的各个结构域的紧密相似的表观结合常数一致。 )乙烷-N,N,N',N'-四乙酸。有限的蛋白水解消化,质谱,动态光散射,尺寸排阻色谱和19 F NMR光谱的结合使我们能够得出有关整体蛋白质折叠和簇形成过程的结论。


























 京公网安备 11010802027423号
京公网安备 11010802027423号