Current Topics in Medicinal Chemistry ( IF 3.4 ) Pub Date : 2020-08-31 , DOI: 10.2174/1568026620666200618114924 Md Tanvir Kabir 1 , Md Sahab Uddin 2, 3 , Ahmed Abdeen 4 , Ghulam Md Ashraf 5, 6 , Asma Perveen 7 , Abdul Hafeez 8 , May N Bin-Jumah 9 , Mohamed M Abdel-Daim 10, 11
|
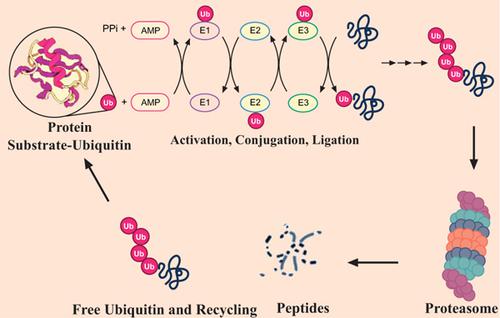
Several proteolytic systems including ubiquitin (Ub)-proteasome system (UPS), chaperonemediated autophagy (CMA), and macroautophagy are used by the mammalian cells to remove misfolded proteins (MPs). UPS mediates degradation of most of the MPs, where Ub-conjugated substrates are deubiquitinated, unfolded, and passed through the proteasome’s narrow chamber, and eventually break into smaller peptides. It has been observed that the substrates that show a specific degradation signal, the KFERQ sequence motif, can be delivered to and go through CMA-mediated degradation in lysosomes. Macroautophagy can help in the degradation of substrates that are prone to aggregation and resistant to both the CMA and UPS. In the aforesaid case, cargoes are separated into autophagosomes before lysosomal hydrolase-mediated degradation. Even though the majority of the aggregated and MPs in the human proteome can be removed via cellular protein quality control (PQC), some mutant and native proteins tend to aggregate into β-sheet-rich oligomers that exhibit resistance to all identified proteolytic processes and can, therefore, grow into extracellular plaques or inclusion bodies. Indeed, the buildup of protease-resistant aggregated and MPs is a usual process underlying various protein misfolding disorders, including neurodegenerative diseases (NDs) for example Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and prion diseases. In this article, we have focused on the contribution of PQC in the degradation of pathogenic proteins in NDs.
中文翻译:

将蛋白质错误折叠与进行性神经退行性疾病的质量控制相关的证据。
哺乳动物细胞使用了几种蛋白水解系统,包括泛素(Ub)-蛋白酶体系统(UPS),伴侣介导的自噬(CMA)和大自噬,以去除错误折叠的蛋白质(MPs)。UPS介导大多数MP的降解,其中结合Ub的底物被去泛素化,展开并穿过蛋白酶体的狭窄腔室,并最终分解为较小的肽。已经观察到,显示特定降解信号,即KFERQ序列基序的底物可以被递送至溶酶体中并通过CMA介导的降解。巨噬细胞自噬可以帮助降解易于聚集并耐CMA和UPS的底物。在上述情况下,在溶酶体水解酶介导的降解之前,将货物分离成自噬体。即使可以通过细胞蛋白质质量控制(PQC)去除人类蛋白质组中的大多数聚集蛋白和MP,某些突变蛋白和天然蛋白也倾向于聚集为富含β-sheet的寡聚体,这些寡聚体对所有已确定的蛋白水解过程均表现出抗性,并且可以因此,会长成细胞外斑块或包涵体。实际上,蛋白酶抗性聚集蛋白和MP的积累是各种蛋白质错误折叠失调的常见过程,包括神经退行性疾病(ND),例如阿尔茨海默氏病,帕金森氏病,亨廷顿氏病,肌萎缩性侧索硬化症和病毒病。在本文中,我们重点研究了PQC在ND中致病蛋白降解中的作用。一些突变蛋白和天然蛋白倾向于聚集为富含β-折叠的寡聚体,这些寡聚体对所有已确定的蛋白水解过程均表现出抗性,因此可以长成细胞外噬菌斑或包涵体。实际上,耐蛋白酶的聚集蛋白和MP的形成是各种蛋白质错误折叠失调的常见过程,包括神经退行性疾病(ND),例如阿尔茨海默氏病,帕金森氏病,亨廷顿氏病,肌萎缩性侧索硬化症和病毒病。在本文中,我们重点研究了PQC在ND中致病蛋白降解中的作用。一些突变蛋白和天然蛋白倾向于聚集为富含β-折叠的寡聚体,这些寡聚体对所有已确定的蛋白水解过程均表现出抗性,因此可以长成细胞外噬菌斑或包涵体。实际上,耐蛋白酶的聚集蛋白和MP的形成是各种蛋白质错误折叠失调的常见过程,包括神经退行性疾病(ND),例如阿尔茨海默氏病,帕金森氏病,亨廷顿氏病,肌萎缩性侧索硬化症和病毒病。在本文中,我们重点研究了PQC在ND中致病蛋白降解中的作用。蛋白酶抗性聚集蛋白和MP的积累是各种蛋白质错误折叠失调的常见过程,包括神经退行性疾病(NDs),例如阿尔茨海默氏病,帕金森氏病,亨廷顿氏病,肌萎缩性侧索硬化症和病毒病。在本文中,我们重点研究了PQC在ND中致病蛋白降解中的作用。蛋白酶抗性聚集蛋白和MP的积累是各种蛋白质错误折叠失调的常见过程,包括神经退行性疾病(NDs),例如阿尔茨海默氏病,帕金森氏病,亨廷顿氏病,肌萎缩性侧索硬化症和病毒病。在本文中,我们重点研究了PQC在ND中致病蛋白降解中的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号