Current Pharmaceutical Biotechnology ( IF 2.8 ) Pub Date : 2020-10-31 , DOI: 10.2174/1389201021666200618161210 Mohammed Al Bratty 1 , Ayman Q Hakami 1 , Hatim A Masmali 1 , Md Shamsher Alam 1 , Hassan A Alhazmi 1 , Neelaveni Thangavel 1 , Asim Najmi 1 , Sivakumar S Moni 2 , Anzarul Haque 3
|
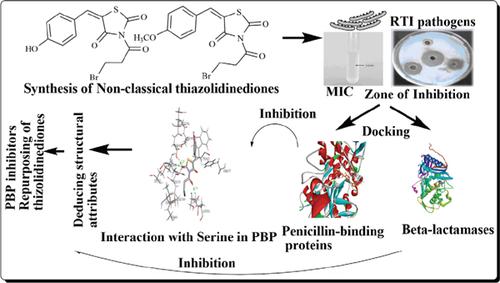
Background and Objectives: Drug design strategies to develop novel broad-spectrum antibacterial agents for the treatment of respiratory tract infections that can combat bacterial resistance are currently gaining momentum. 2,4-thiazolidinedione is a structural scaffold that contains pharmacophores similar to β-lactam and non- β-lactam antibiotics. The objective of the study was to synthesize newer 3,5-Disubstituted-2,4-Thiazolidinediones (DTZDs) and subject them to in vitro antibacterial screening against bacterial pathogens. Also, we performed in silico docking of selected compounds to penicillin-binding proteins and beta-lactamases.
Methods: Intermediate Schiff bases were prepared by the reaction between 2,4-thiazolidinedione and an appropriate aldehyde followed by acylation of the ring nitrogen with 3-brompropanoyl chloride resulting in DTZDs. Minimum inhibitory concentrations were determined against few bacteria infecting the respiratory tract by the broth tube dilution method. Zones of inhibitions against the bacteria were also determined using agar well diffusion technique. Molecular docking of the compounds to all types of Penicillin-Binding Proteins (PBPs) and β-lactamases was also carried out.
Results: Compounds DTZD12 and DTZD16 exhibited broad-spectrum antibacterial activity. The minimum inhibitory concentrations of the compounds were 175μg/100μL. Measurements of the zones of inhibitions indicated that compound DTZD12 was more active than DZTD16. E. coli was the most susceptible organism. Docking results established that both the compounds were able to interact with PBPs and β-lactamases through strong hydrogen bonds, especially the unique interaction with active serine residue of the PBP for inhibition of cell wall synthesis.
Conclusion: DTZD12 and DTZD16 can be developed into antibacterial drugs for respiratory tract infections to oppose bacterial resistance, or can also be used as leads for repurposing the existing 2,4- thiazolidinediones.
中文翻译:

噻唑烷二酮类抗呼吸道病原菌的谱:体外和计算机方法。
背景和目的:开发用于治疗呼吸道感染的新型广谱抗菌剂的药物设计策略目前正在获得动力。2,4-噻唑烷二酮是一种结构支架,含有类似于β-内酰胺和非β-内酰胺抗生素的药效团。该研究的目的是合成更新的 3,5-二取代-2,4-噻唑烷二酮 (DTZD),并对它们进行体外抗菌筛选以对抗细菌病原体。此外,我们还对选定的化合物与青霉素结合蛋白和 β-内酰胺酶进行了计算机对接。
方法:通过 2,4-噻唑烷二酮和适当的醛反应制备中间体席夫碱,然后用 3-溴丙酰氯酰化环氮,生成 DTZD。用肉汤管稀释法测定感染呼吸道的少数细菌的最低抑菌浓度。还使用琼脂孔扩散技术确定了对细菌的抑制区域。还进行了化合物与所有类型的青霉素结合蛋白 (PBP) 和 β-内酰胺酶的分子对接。
结果:化合物DTZD12和DTZD16表现出广谱抗菌活性。化合物的最低抑菌浓度为175μg/100μL。抑制区的测量表明化合物 DTZD12 比 DZTD16 更有活性。大肠杆菌是最敏感的生物。对接结果表明,这两种化合物都能够通过强氢键与 PBP 和 β-内酰胺酶相互作用,尤其是与 PBP 的活性丝氨酸残基的独特相互作用,从而抑制细胞壁合成。
结论:DTZD12和DTZD16可开发成呼吸道感染的抗菌药物,对抗细菌耐药性,也可作为现有2,4-噻唑烷二酮类药物再利用的先导物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号