Current Organic Synthesis ( IF 1.8 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570179417666200615153536 Shailesh Singh 1 , Jyoti Tiwari 2 , Deepali Jaiswal 2 , Amit Kumar Sharma 2 , Jaya Singh 3 , Vandana Singh 1 , Jagdamba Singh 2
|
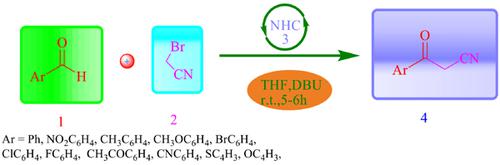
Background: A novel one-pot N-heterocyclic carbene (NHC)-catalysed acylation of 2- bromoacetonitrile with aromatic aldehydes is reported. The protocol involves carbonyl umpolung reactivity of aldehydes in which the carbonyl carbon attacks nucleophilically (as d1 nucleophile) on the electrophilic terminal of 2-bromoacetonitrile to afford 3-aryl-3-oxopropanenitrile. The salient features of this procedure are short reaction time, operational simplicity, ambient temperature, no by-product formation and high yields.
Materials and Methods: A flame-dried round bottom flask was charged with Imidazolium salts (3a) (0.20 mmol). Aldehyde 1a (1.0 mmol), 2-bromoacetonitrile 2 (1.0 mmol), and THF / t-BuOH 5 mL; 10:1) were added at positive nitrogen pressure followed by the addition of DBU (0.15 mmol) through stirring. The resulting yellow- orange solution was stirred at room temperature for 5-6 h. After completion of the reaction (TLC monitored), the reaction mixture was concentrated under reduced pressure. The product was purified using hexane / EtOAc (10:1) as an eluent to provide analytically pure compound 4a. Physical data of representative compounds and the NMR spectroscopic data are in agreement with the literature value.
Results and Discussion: The salient features of this procedure are short reaction time, operational simplicity, ambient temperature, no by-product formation and high yields.
Conclusion: To sum up, we have developed a convenient, efficient and one-pot route for 3-oxo-3- phenylpropanenitrile synthesis from NHC promoted direct nucleophilic acylation of aromatic aldehydes using 2- bromoacetonitrile. This method provided a wide range of products and good yields. To best of our knowledge, this is the new report for the synthesis of 3-oxo-3-phenylpropanenitrile through NHC promoted nucleophilic acylation of aromatic aldehyde.
中文翻译:

用芳族醛进行亲核酰化反应生成2个溴乙腈:合成活性亚甲基化合物的主要策略。
背景:报道了一种新型的一锅N-杂环卡宾(NHC)催化的2-溴乙腈与芳族醛的酰化反应。该规程涉及醛的羰基氨酚反应性,其中羰基碳亲核(如d1亲核体)攻击2-溴乙腈的亲电子端,得到3-芳基-3-氧代丙烷腈。该方法的显着特征是反应时间短,操作简便,环境温度高,无副产物形成和产率高。
材料和方法:在火焰干燥的圆底烧瓶中装入咪唑鎓盐(3a)(0.20 mmol)。醛1a(1.0 mmol),2-溴乙腈2(1.0 mmol)和THF / t-BuOH 5 mL; 在正氮气压力下加入10∶1),然后通过搅拌加入DBU(0.15mmol)。将所得的黄橙色溶液在室温下搅拌5-6小时。反应完成后(监控TLC),将反应混合物减压浓缩。使用己烷/ EtOAc(10:1)作为洗脱剂纯化产物,以提供分析纯的化合物4a。代表性化合物的物理数据和NMR光谱数据与文献值一致。
结果与讨论:该方法的主要特点是反应时间短,操作简便,环境温度高,无副产物形成和高收率。
结论:综上所述,我们已经开发了一种便捷,有效且一锅法的方法,该方法可通过NHC促进2-氧代乙腈对芳香族醛的直接亲核酰化反应来合成3-氧代-3-苯基丙腈。该方法提供了广泛的产品和良好的产率。据我们所知,这是通过NHC促进芳族醛的亲核酰化反应合成3-氧代-3-苯基丙腈的新报告。


























 京公网安备 11010802027423号
京公网安备 11010802027423号