Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Al+ (C6H6)13: The Origin of Magic Number in Metal–Benzene Clusters Determined by the Nature of the Core
CCS Chemistry ( IF 11.2 ) Pub Date : 2019-11-02 , DOI: 10.31635/ccschem.019.20190033 Hanyu Zhang 1 , Arthur C. Reber 2 , Lijun Geng 1 , Daniel Rabayda 2 , Haiming Wu 1 , Zhixun Luo 1 , Jiannian Yao 1 , Shiv N. Khanna 2
CCS Chemistry ( IF 11.2 ) Pub Date : 2019-11-02 , DOI: 10.31635/ccschem.019.20190033 Hanyu Zhang 1 , Arthur C. Reber 2 , Lijun Geng 1 , Daniel Rabayda 2 , Haiming Wu 1 , Zhixun Luo 1 , Jiannian Yao 1 , Shiv N. Khanna 2
Affiliation
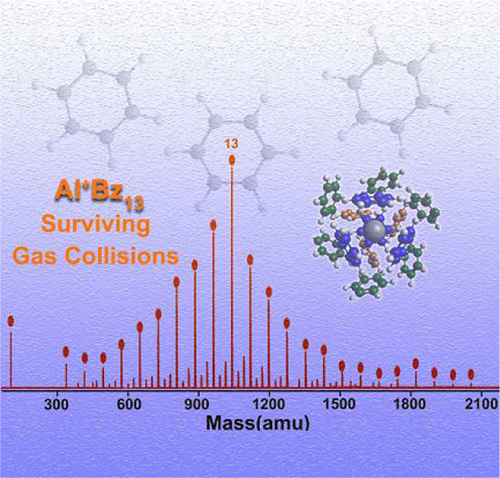
|
The identification of highly abundant, “magic” species in the mass spectra of clusters have proven to be
valuable in nanoscience, leading to the discovery of
new stable species such as fullerenes and the electronic shell structures of metallic clusters. However,
identifying “magic” clusters formed via noncovalent
interactions faces challenges of poor clustering
and difficulties in structure determination. Using a
customized ultrafast, deep-ultraviolet laser ionization mass spectrometer, we report the finding of
aluminum–benzene complex (Al+
Bz13; Bz = benzene)
as a magic cluster in the spectra of Al+
Bzn (n ≤ 40)
clusters, and herewith compared with the mass spectra and stability of vanadium–benzene complex
(V+
Bzn; n ≤ 40) clusters. Theoretical investigations,
using Amsterdam Density Functional with dispersion
correction, identified the structure of the dominant
Al+
Bz13 and revealed that the origin of the magic
number Al+
Bz13 is due to the geometric structure of
Bzn shell enclosure of Al+
, contrary to the V+
Bzn
clusters in which Bzn molecules formed a sandwich
complex with the V+ core in V+
Bz2. The differences in
the structures of the clusters became apparent from
their distinct characteristic features, revealed by Bz
as a solvent around Al+ being strikingly different from
the observed Bz solvent shell for V+
, attributable to
the variances in solvation numbers of 3 or 4 for
aluminum and 2 for vanadium. These results provide
new details into coordination chemistry and a
strategy to dissect the interactions of metal ions in
solvents like benzene.
中文翻译:

Al +(C6H6)13的形成:核心性质决定的金属-苯簇中的幻数起源
在团簇的质谱图中鉴定高度丰富的“魔术”物种已被证明在纳米科学中具有重要价值,从而导致发现了新的稳定物种,例如富勒烯和金属团簇的电子壳结构。然而,识别通过非共价相互作用形成的“魔术”簇面临着簇聚困难和结构确定困难的挑战。使用定制的超快速深紫外激光电离质谱仪,我们报告了在Al + Bzn(n≤40)团簇的光谱中发现了铝-苯配合物(Al + Bz13; Bz =苯)作为魔术团簇,并且因此,与钒-苯配合物(V + Bzn; n≤40)簇的质谱和稳定性进行了比较。理论研究,使用阿姆斯特丹色散函数和色散校正,鉴定了主要Al + Bz13的结构,并揭示了魔数Al + Bz13的起源是由于Al +的Bzn壳包围的几何结构,与V + Bzn团簇相反,在该簇中,Bzn分子与V +核形成了三明治复合物在V + Bz2中。簇的结构差异从其独特的特征中变得明显,Bz揭示了Al +周围的溶剂与V +观察到的Bz溶剂壳明显不同,这归因于铝和铝的溶剂化数分别为3或4。 2为钒。这些结果为配位化学提供了新的细节,并提供了一种分析诸如苯之类的溶剂中金属离子相互作用的策略。
更新日期:2020-06-24
中文翻译:

Al +(C6H6)13的形成:核心性质决定的金属-苯簇中的幻数起源
在团簇的质谱图中鉴定高度丰富的“魔术”物种已被证明在纳米科学中具有重要价值,从而导致发现了新的稳定物种,例如富勒烯和金属团簇的电子壳结构。然而,识别通过非共价相互作用形成的“魔术”簇面临着簇聚困难和结构确定困难的挑战。使用定制的超快速深紫外激光电离质谱仪,我们报告了在Al + Bzn(n≤40)团簇的光谱中发现了铝-苯配合物(Al + Bz13; Bz =苯)作为魔术团簇,并且因此,与钒-苯配合物(V + Bzn; n≤40)簇的质谱和稳定性进行了比较。理论研究,使用阿姆斯特丹色散函数和色散校正,鉴定了主要Al + Bz13的结构,并揭示了魔数Al + Bz13的起源是由于Al +的Bzn壳包围的几何结构,与V + Bzn团簇相反,在该簇中,Bzn分子与V +核形成了三明治复合物在V + Bz2中。簇的结构差异从其独特的特征中变得明显,Bz揭示了Al +周围的溶剂与V +观察到的Bz溶剂壳明显不同,这归因于铝和铝的溶剂化数分别为3或4。 2为钒。这些结果为配位化学提供了新的细节,并提供了一种分析诸如苯之类的溶剂中金属离子相互作用的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号