当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nitrogen-Walk Approach to Explore Bioisosteric Replacements in a Series of Potent A2B Adenosine Receptor Antagonists.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.jmedchem.0c00564 Ana Mallo-Abreu , Rubén Prieto-Díaz , Willem Jespers 1 , Jhonny Azuaje , Maria Majellaro , Carmen Velando , Xerardo García-Mera , Olga Caamaño , José Brea 2 , María I Loza 2 , Hugo Gutiérrez-de-Terán 1 , Eddy Sotelo
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.jmedchem.0c00564 Ana Mallo-Abreu , Rubén Prieto-Díaz , Willem Jespers 1 , Jhonny Azuaje , Maria Majellaro , Carmen Velando , Xerardo García-Mera , Olga Caamaño , José Brea 2 , María I Loza 2 , Hugo Gutiérrez-de-Terán 1 , Eddy Sotelo
Affiliation
|
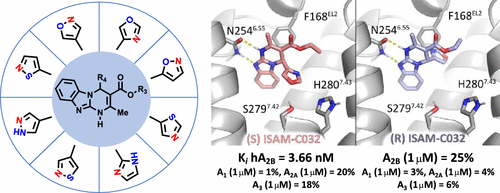
A systematic exploration of bioisosteric replacements for furan and thiophene cores in a series of potent A2BAR antagonists has been carried out using the nitrogen-walk approach. A collection of 42 novel alkyl 4-substituted-2-methyl-1,4-dihydrobenzo[4,5]imidazo[1,2-a]pyrimidine-3-carboxylates, which contain 18 different pentagonal heterocyclic frameworks at position 4, was synthesized and evaluated. This study enabled the identification of new ligands that combine remarkable affinity (Ki < 30 nM) and exquisite selectivity. The structure–activity relationship (SAR) trends identified were substantiated by a molecular modeling study, based on a receptor-driven docking model and including a systematic free energy perturbation (FEP) study. Preliminary evaluation of the CYP3A4 and CYP2D6 inhibitory activity in optimized ligands evidenced weak and negligible activity, respectively. The stereospecific interaction between hA2BAR and the eutomer of the most attractive novel antagonist (S)-18g (Ki = 3.66 nM) was validated.
中文翻译:

氮步行法探索一系列有效的A2B腺苷受体拮抗剂的生物等效取代。
使用氮游走法已经对一系列有效的A 2B AR拮抗剂中的呋喃和噻吩核的生物立体替代物进行了系统的研究。收集了42种新颖的4-取代-2-甲基-1,4-二氢苯并[4,5]咪唑并[1,2 - a ]嘧啶-3-羧酸烷基酯,它们在4位含有18个不同的五角杂环骨架。综合和评估。这项研究使得能够鉴定结合了显着亲和力(K i<30 nM)和精湛的选择性。基于受体驱动的对接模型并包括系统的自由能扰动(FEP)研究,通过分子建模研究证实了所确定的构效关系(SAR)趋势。在优化配体中对CYP3A4和CYP2D6抑制活性的初步评估分别显示弱和可忽略的活性。验证了h A 2B AR与最有吸引力的新型拮抗剂(S)-18g(K i = 3.66 nM)的eutomer之间的立体定向相互作用。
更新日期:2020-07-23
中文翻译:

氮步行法探索一系列有效的A2B腺苷受体拮抗剂的生物等效取代。
使用氮游走法已经对一系列有效的A 2B AR拮抗剂中的呋喃和噻吩核的生物立体替代物进行了系统的研究。收集了42种新颖的4-取代-2-甲基-1,4-二氢苯并[4,5]咪唑并[1,2 - a ]嘧啶-3-羧酸烷基酯,它们在4位含有18个不同的五角杂环骨架。综合和评估。这项研究使得能够鉴定结合了显着亲和力(K i<30 nM)和精湛的选择性。基于受体驱动的对接模型并包括系统的自由能扰动(FEP)研究,通过分子建模研究证实了所确定的构效关系(SAR)趋势。在优化配体中对CYP3A4和CYP2D6抑制活性的初步评估分别显示弱和可忽略的活性。验证了h A 2B AR与最有吸引力的新型拮抗剂(S)-18g(K i = 3.66 nM)的eutomer之间的立体定向相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号