当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Air-Stable Calcium Cyanamide-Supported Ruthenium Catalyst for Ammonia Synthesis and Decomposition
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-06-23 00:00:00 , DOI: 10.1021/acsaem.0c00754 Kazuhisa Kishida 1 , Masaaki Kitano 1, 2 , Masato Sasase 1 , Peter V. Sushko 3 , Hitoshi Abe 4, 5, 6 , Yasuhiro Niwa 4 , Kiya Ogasawara 1 , Toshiharu Yokoyama 1 , Hideo Hosono 1
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-06-23 00:00:00 , DOI: 10.1021/acsaem.0c00754 Kazuhisa Kishida 1 , Masaaki Kitano 1, 2 , Masato Sasase 1 , Peter V. Sushko 3 , Hitoshi Abe 4, 5, 6 , Yasuhiro Niwa 4 , Kiya Ogasawara 1 , Toshiharu Yokoyama 1 , Hideo Hosono 1
Affiliation
|
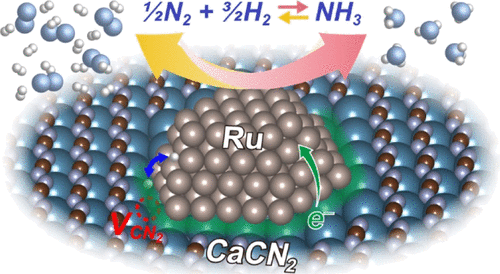
Efficient ammonia synthesis and decomposition processes under mild conditions are important to meet the expanding demand in major applications of ammonia as the energy carrier and to provide feedstock for chemical industry. Here, we report that air-stable calcium cyanamide-supported ruthenium (Ru/CaCN2) works as an efficient and stable catalyst for ammonia synthesis and decomposition. Ru/CaCN2 exhibits greater catalytic performances for both reactions than Ru/Ca2N electride and Ru–Cs/MgO. The kinetic analysis for ammonia synthesis suggests that Ru/CaCN2 exhibits low apparent activation energy and high resistance to hydrogen poisoning, which has characteristics similar to the kinetic parameters of Ru-supported electride catalyst. H2-temperature programmed reaction and temperature programmed desorption revealed that Ru promoted the formation of CN2 vacancies on the CaCN2 surface which in turn capture hydrogen as H– ions during the reaction. Density functional theory calculations provide insights into how the formation of CN2 vacancies is promoted by Ru, which leads to the decrease in the work function of the CaCN2 surface and the hydrogen capture at the Ru-support interface. These results suggest that the high catalytic performance of Ru/CaCN2 can be attributed to the formation of a quasi-electride structure at the Ru–CaCN2 interface.
中文翻译:

空气稳定的氰氨化钙负载钌催化剂用于氨的合成和分解
在温和的条件下有效的氨合成和分解过程对于满足氨作为能源载体的主要应用中不断增长的需求以及为化学工业提供原料至关重要。在这里,我们报告说,空气稳定的氰氨化钙负载钌(Ru / CaCN 2)是合成氨和分解氨的有效且稳定的催化剂。Ru / CaCN 2在两个反应中均显示出比Ru / Ca 2 N氮化物和Ru-Cs / MgO更高的催化性能。对氨合成的动力学分析表明,Ru / CaCN 2表现出低的表观活化能和高的抗氢中毒性,其特性类似于Ru负载的电子催化剂的动力学参数。H2 -温度控制的反应和程序升温脱附表明,钌促进CN的形成2的CACN上空缺2表面继而捕获氢以H -在反应过程中的离子。密度泛函理论计算提供了有关Ru如何促进CN 2空位形成的见解,这导致CaCN 2表面的功函数降低以及Ru-载体界面处的氢捕获。这些结果表明,Ru / CaCN 2的高催化性能可以归因于在Ru–CaCN 2界面处形成准电氮化物结构。
更新日期:2020-06-23
中文翻译:

空气稳定的氰氨化钙负载钌催化剂用于氨的合成和分解
在温和的条件下有效的氨合成和分解过程对于满足氨作为能源载体的主要应用中不断增长的需求以及为化学工业提供原料至关重要。在这里,我们报告说,空气稳定的氰氨化钙负载钌(Ru / CaCN 2)是合成氨和分解氨的有效且稳定的催化剂。Ru / CaCN 2在两个反应中均显示出比Ru / Ca 2 N氮化物和Ru-Cs / MgO更高的催化性能。对氨合成的动力学分析表明,Ru / CaCN 2表现出低的表观活化能和高的抗氢中毒性,其特性类似于Ru负载的电子催化剂的动力学参数。H2 -温度控制的反应和程序升温脱附表明,钌促进CN的形成2的CACN上空缺2表面继而捕获氢以H -在反应过程中的离子。密度泛函理论计算提供了有关Ru如何促进CN 2空位形成的见解,这导致CaCN 2表面的功函数降低以及Ru-载体界面处的氢捕获。这些结果表明,Ru / CaCN 2的高催化性能可以归因于在Ru–CaCN 2界面处形成准电氮化物结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号