当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heat shock protein 90 enhances the electron transfer between the FMN and heme cofactors in neuronal nitric oxide synthase
FEBS Letters ( IF 3.5 ) Pub Date : 2020-07-04 , DOI: 10.1002/1873-3468.13870 Huayu Zheng 1, 2 , Jinghui Li 1 , Changjian Feng 1, 2
FEBS Letters ( IF 3.5 ) Pub Date : 2020-07-04 , DOI: 10.1002/1873-3468.13870 Huayu Zheng 1, 2 , Jinghui Li 1 , Changjian Feng 1, 2
Affiliation
|
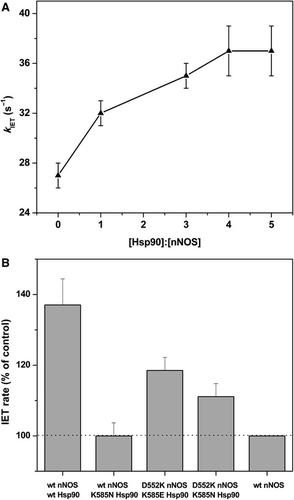
Heat shock protein 90 (Hsp90) is a key regulator of nitric oxide synthase (NOS) in vivo. Despite its functional importance, little is known about the underlying molecular mechanism. Here, purified dimeric human Hsp90α was used to investigate whether (and if so, how) Hsp90 affects the FMN–heme interdomain electron transfer (IET) step in NOS. Hsp90α increases the IET rate for rat neuronal NOS (nNOS) in a dose‐saturable manner, and a single charge‐neutralization mutation at conserved Hsp90 K585 abolishes the effect. The kinetic results with added Ficoll 70, a crowder, further indicate that Hsp90 enhances the FMN–heme IET through specific association with nNOS. The Hsp90‐nNOS docking models provide hints on the putative role of Hsp90 in constraining the available conformational space for the FMN domain motions.
中文翻译:

热休克蛋白 90 增强神经元一氧化氮合酶中 FMN 和血红素辅因子之间的电子转移
热休克蛋白 90 (Hsp90) 是体内一氧化氮合酶 (NOS) 的关键调节因子。尽管其功能很重要,但对其潜在的分子机制知之甚少。在这里,纯化的二聚体人 Hsp90α 用于研究 Hsp90 是否(如果是,如何)影响 NOS 中的 FMN-血红素域间电子转移 (IET) 步骤。Hsp90α 以剂量可饱和的方式增加大鼠神经元 NOS (nNOS) 的 IET 率,保守的 Hsp90 K585 上的单个电荷中和突变消除了这种作用。添加 Ficoll 70 的动力学结果进一步表明 Hsp90 通过与 nNOS 的特定关联增强 FMN-血红素 IET。Hsp90-nNOS 对接模型暗示了 Hsp90 在限制 FMN 域运动的可用构象空间方面的假定作用。
更新日期:2020-07-04
中文翻译:

热休克蛋白 90 增强神经元一氧化氮合酶中 FMN 和血红素辅因子之间的电子转移
热休克蛋白 90 (Hsp90) 是体内一氧化氮合酶 (NOS) 的关键调节因子。尽管其功能很重要,但对其潜在的分子机制知之甚少。在这里,纯化的二聚体人 Hsp90α 用于研究 Hsp90 是否(如果是,如何)影响 NOS 中的 FMN-血红素域间电子转移 (IET) 步骤。Hsp90α 以剂量可饱和的方式增加大鼠神经元 NOS (nNOS) 的 IET 率,保守的 Hsp90 K585 上的单个电荷中和突变消除了这种作用。添加 Ficoll 70 的动力学结果进一步表明 Hsp90 通过与 nNOS 的特定关联增强 FMN-血红素 IET。Hsp90-nNOS 对接模型暗示了 Hsp90 在限制 FMN 域运动的可用构象空间方面的假定作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号