当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
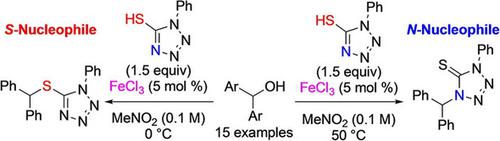
Chemodivergent Dehydrative Nucleophilic Substitutions of Diarylmethanols with 1‐Phenyl‐1H‐tetrazole‐5‐thiol Catalyzed by FeCl3
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-06-23 , DOI: 10.1002/ajoc.202000301 Ryoga Oda 1 , Kenya Nakata 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-06-23 , DOI: 10.1002/ajoc.202000301 Ryoga Oda 1 , Kenya Nakata 1
Affiliation
|
Chemodivergent dehydrative nucleophilic substitutions of diarylmethanols with 1‐phenyl‐1H‐tetrazole‐5‐thiol were achieved using FeCl3 as a catalyst in MeNO2 by changing reaction temperature. It was found that sulfur nucleophilic attack occurred to afford kinetically controlled products that rearranged into thermodynamically controlled products. A variety of diarylmethanols could be applied to the reaction, irrespective of the substituents on the aromatic rings of the substrates. It was also revealed that the reactions of several kinds of alkyl‐ and heteroaryl‐substituted benzyl alcohols gave moderate to good product yields. The controlled experiments performed revealed that both reactions proceeded in an SN1 fashion and heating with a Lewis acid was necessary for rearrangement.
中文翻译:

FeCl3催化二芳基甲醇的化学发散脱水亲核取代基与1-苯基-1H-四唑-5-硫醇
通过使用FeCl 3作为催化剂在MeNO 2中通过改变反应温度实现二芳基甲醇被1-苯基-1 H-四唑-5-硫醇的化学扩散脱水亲核取代。发现发生了硫亲核攻击,得到了动力学控制的产物,该产物被重排为热力学控制的产物。可以将多种二芳基甲醇应用于该反应,而与底物芳环上的取代基无关。还表明,几种烷基和杂芳基取代的苄醇的反应产生中等至良好的产物收率。进行的对照实验表明,两个反应均在S N中进行1重排方法和路易斯酸加热是必需的。
更新日期:2020-08-08
中文翻译:

FeCl3催化二芳基甲醇的化学发散脱水亲核取代基与1-苯基-1H-四唑-5-硫醇
通过使用FeCl 3作为催化剂在MeNO 2中通过改变反应温度实现二芳基甲醇被1-苯基-1 H-四唑-5-硫醇的化学扩散脱水亲核取代。发现发生了硫亲核攻击,得到了动力学控制的产物,该产物被重排为热力学控制的产物。可以将多种二芳基甲醇应用于该反应,而与底物芳环上的取代基无关。还表明,几种烷基和杂芳基取代的苄醇的反应产生中等至良好的产物收率。进行的对照实验表明,两个反应均在S N中进行1重排方法和路易斯酸加热是必需的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号