当前位置:
X-MOL 学术
›
Adv. Electron. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Correlating the Structural and Photophysical Properties of Ortho, Meta, and Para‐Carboranyl–Anthracene Dyads
Advanced Electronic Materials ( IF 6.2 ) Pub Date : 2020-06-23 , DOI: 10.1002/aelm.202000312 Adam V. Marsh 1 , Matthew J. Dyson 2 , Nathan J. Cheetham 3 , Matthew Bidwell 1 , Mark Little 1 , Andrew J. P. White 1 , Colin N. Warriner 4 , Anthony C. Swain 4 , Iain McCulloch 5 , Paul N. Stavrinou 6 , Stefan C. J. Meskers 2 , Martin Heeney 1
Advanced Electronic Materials ( IF 6.2 ) Pub Date : 2020-06-23 , DOI: 10.1002/aelm.202000312 Adam V. Marsh 1 , Matthew J. Dyson 2 , Nathan J. Cheetham 3 , Matthew Bidwell 1 , Mark Little 1 , Andrew J. P. White 1 , Colin N. Warriner 4 , Anthony C. Swain 4 , Iain McCulloch 5 , Paul N. Stavrinou 6 , Stefan C. J. Meskers 2 , Martin Heeney 1
Affiliation
|
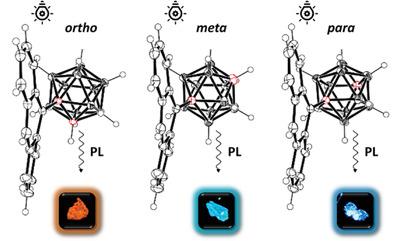
The role of the carborane isomer is investigated on the structural and photophysical properties of molecules comprising a carborane cluster and a conjugated organic moiety is investigated by synthesizing isomeric o‐, m‐, and p‐carboranyl‐anthracene donor–acceptor dyads. While appending a carborane leads to emission from a low energy intramolecular charge transfer state for the o‐isomer, as well as emission from an excited state localized on the anthracene, this is not the case for the m‐ and p‐carborane derivatives. This difference is attributed to a lower electron affinity for the latter two isomers. However, adding both m‐ and p‐ deforms the aromatic backbone and increases its structural rigidity, reducing non‐radiative decay pathways and hence enhancing photoluminescence quantum efficiency relative to anthracene.
中文翻译:

关联邻,间和对-碳硼烷基蒽蒽的结构和光物理性质
异构体在包括碳硼烷簇和共轭有机部分的分子的结构和光物理性质研究了碳硼烷的作用是通过合成的异构体研究ö, -米- ,和p -carboranyl蒽供体-受体成对层。而从用于低能量分子内电荷转移状态追加一个碳硼烷通向排放ø -异构体,以及从定位于蒽激发态发射,这是不适合的情况下米-和p -carborane衍生物。该差异归因于对后两种异构体的较低的电子亲和力。但是,同时添加m-和p‐使芳族主链变形并增加其结构刚度,减少非辐射衰变途径,从而相对于蒽提高光致发光量子效率。
更新日期:2020-08-10
中文翻译:

关联邻,间和对-碳硼烷基蒽蒽的结构和光物理性质
异构体在包括碳硼烷簇和共轭有机部分的分子的结构和光物理性质研究了碳硼烷的作用是通过合成的异构体研究ö, -米- ,和p -carboranyl蒽供体-受体成对层。而从用于低能量分子内电荷转移状态追加一个碳硼烷通向排放ø -异构体,以及从定位于蒽激发态发射,这是不适合的情况下米-和p -carborane衍生物。该差异归因于对后两种异构体的较低的电子亲和力。但是,同时添加m-和p‐使芳族主链变形并增加其结构刚度,减少非辐射衰变途径,从而相对于蒽提高光致发光量子效率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号