Cell Calcium ( IF 4 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.ceca.2020.102248 Peterson Anand 1 , Alan G S Harper 2
|
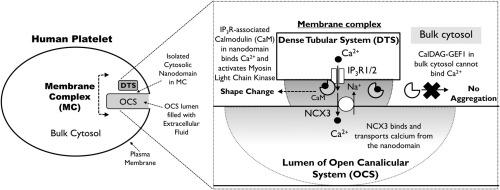
Human platelets use a rise in cytosolic Ca2+ concentration to activate all stages of thrombus formation, however, how they are able to decode cytosolic Ca2+ signals to trigger each of these independently is unknown. Other cells create local Ca2+ signals to activate Ca2+-sensitive effectors specifically localised to these subcellular regions. However, no previous study has demonstrated that agonist-stimulated human platelets can generate a local cytosolic Ca2+ signal. Platelets possess a structure called the membrane complex (MC) where the main intracellular calcium store, the dense tubular system (DTS), is coupled tightly to an invaginated portion of the plasma membrane called the open canalicular system (OCS). Here we hypothesised that human platelets use a Ca2+ nanodomain created within the MC to control the earliest phases of platelet activation. Dimethyl-BAPTA-loaded human platelets were stimulated with thrombin in the absence of extracellular Ca2+ to isolate a cytosolic Ca2+ nanodomain created by Ca2+ release from the DTS. In the absence of any detectable rise in global cytosolic Ca2+ concentration, thrombin stimulation triggered Na+/Ca2+ exchanger (NCX)-dependent Ca2+ removal into the extracellular space, as well as Ca2+-dependent shape change in the absence of platelet aggregation. The NCX-mediated Ca2+ removal was dependent on the normal localisation of the DTS, and immunofluorescent staining of NCX3 demonstrated its localisation to the OCS, consistent with this Ca2+ nanodomain being formed within the MC. These results demonstrated that human platelets possess a functional Ca2+ nanodomain contained within the MC that can control shape change independently of platelet aggregation.
中文翻译:

人血小板使用胞质 Ca2+ 纳米域来激活 Ca2+ 依赖性形状变化,而与血小板聚集无关。
人血小板利用细胞溶质 Ca 2+浓度的升高来激活血栓形成的所有阶段,然而,它们如何能够解码细胞溶质 Ca 2+信号以独立触发这些信号中的每一个尚不清楚。其他细胞产生局部Ca 2+信号以激活特异性定位于这些亚细胞区域的Ca 2+敏感效应物。然而,之前没有研究表明激动剂刺激的人血小板可以产生局部细胞溶质 Ca 2+信号。血小板具有称为膜复合体 (MC) 的结构,其中主要的细胞内钙储存,即致密管系统 (DTS),与称为开放小管系统 (OCS) 的质膜内陷部分紧密耦合。在这里,我们假设人类血小板使用在 MC 内创建的 Ca 2+纳米域来控制血小板活化的最早阶段。二甲基BAPTA加载的人血小板在不存在外Ca与刺激凝血酶2+以分离细胞内的Ca 2+以Ca创建nanodomain 2+从DTS释放。在没有任何可检测到的整体胞质 Ca 2+浓度升高的情况下,凝血酶刺激触发了 Na +/Ca 2+交换剂 (NCX) 依赖的 Ca 2+去除到细胞外空间,以及在没有血小板聚集的情况下Ca 2+依赖的形状变化。NCX 介导的 Ca 2+去除取决于 DTS 的正常定位,NCX3 的免疫荧光染色表明其定位于 OCS,与在 MC 内形成的Ca 2+纳米域一致。这些结果表明,人血小板具有包含在 MC 内的功能性 Ca 2+纳米域,可以独立于血小板聚集控制形状变化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号