Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.bbamem.2020.183403 Takashi Ohgita 1 , Yuki Takechi-Haraya 2 , Keisuke Okada 1 , Saki Matsui 1 , Misaki Takeuchi 1 , Chihiro Saito 3 , Kazuchika Nishitsuji 4 , Kenji Uchimura 5 , Ryuji Kawano 3 , Koki Hasegawa 6 , Kumiko Sakai-Kato 7 , Kenichi Akaji 8 , Ken-Ichi Izutsu 2 , Hiroyuki Saito 1
|
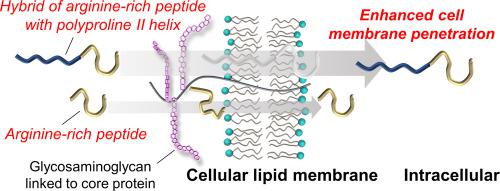
The left-handed, extended polyproline II (PPII) helix is a unique secondary structure which potently modulates peptide/protein functions through its constraint conformation. To investigate the effect of PPII helix on the direct cell membrane penetration of arginine-rich peptides, we designed a polyproline-containing arginine-rich peptide P9R7W (PPPPPPPPPRRRRRRRW) by introducing nine proline residues into a linear R7W (RRRRRRRW) peptide. Circular dichroism spectroscopy showed that P9R7W has the PPII helix structure in solution whereas R7W is predominantly in random coil structure. Tryptophan fluorescence measurements demonstrated that P9R7W binds to negatively charged lipid vesicles with similar affinity to R7W, in which there was no significant change in the PPII helix structure. Flow cytometry and confocal laser scanning microscopy analyses showed that P9R7W has an ability to penetrate into CHO-K1 cells more efficiently compared to R7W with no cytotoxicity. Consistently, a channel current analysis unveiled that P9R7W penetrates planar lipid bilayer membranes more efficiently than R7W without significant membrane perturbation. Our results indicate that the PPII helix structure can enhance the membrane penetration efficiency of arginine-rich peptides without lipid membrane perturbation.
中文翻译:

聚脯氨酸II螺旋结构增强了富含精氨酸的肽的直接膜渗透。
左旋延伸的脯氨酸II(PPII)螺旋是独特的二级结构,可通过其限制性构象有效地调节肽/蛋白质功能。为了研究PPII螺旋对富含精氨酸的肽直接细胞膜渗透的影响,我们通过将9个脯氨酸残基引入线性R7W(RRRRRRRW)肽中,设计了一种含多脯氨酸的富含精氨酸的肽P9R7W(PPPPPPPPPRRRRRRRW)。圆二色光谱表明,P9R7W在溶液中具有PPII螺旋结构,而R7W主要在无规卷曲结构中。色氨酸荧光测量表明,P9R7W以与R7W相似的亲和力与带负电荷的脂质小泡结合,其中PPII螺旋结构没有明显变化。流式细胞术和共聚焦激光扫描显微镜分析表明,与没有细胞毒性的R7W相比,P9R7W具有更有效地穿透CHO-K1细胞的能力。一致地,通道电流分析显示,P9R7W比R7W更有效地穿透平面脂质双层膜,而没有明显的膜扰动。我们的结果表明,PPII螺旋结构可以提高富含精氨酸的肽的膜渗透效率,而不会引起脂质膜的扰动。



























 京公网安备 11010802027423号
京公网安备 11010802027423号