当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
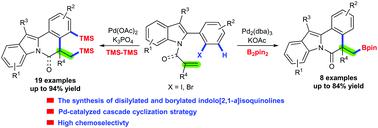
Palladium-catalyzed domino Heck-disilylation and -borylation of alkene-tethered 2-(2-halophenyl)-1H-indoles: access to diverse disilylated and borylated indolo[2,1-a]isoquinolines
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0qo00492h Haiyan Lu 1, 2, 3, 4, 5 , Xiumei Yang 1, 2, 3, 4, 5 , Liwei Zhou 1, 2, 3, 4, 5 , Wenguang Li 1, 2, 3, 4, 5 , Guobo Deng 1, 2, 3, 4, 5 , Yuan Yang 1, 2, 3, 4, 5 , Yun Liang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0qo00492h Haiyan Lu 1, 2, 3, 4, 5 , Xiumei Yang 1, 2, 3, 4, 5 , Liwei Zhou 1, 2, 3, 4, 5 , Wenguang Li 1, 2, 3, 4, 5 , Guobo Deng 1, 2, 3, 4, 5 , Yuan Yang 1, 2, 3, 4, 5 , Yun Liang 1, 2, 3, 4, 5
Affiliation
|
The chemoselective reaction remains a tremendous but particularly fascinating challenge in organic synthesis. Herein, we report a new domino Heck/disilylation reaction of alkene-tethered 2-(2-halophenyl)-1H-indoles with hexamethyldisilane under palladium catalysis. This procedure involving the formation of one C–C bond and two C–Si bonds proceeds efficiently through two key steps of the chemoselective Heck reaction and C–H activation by overcoming undesirable C3–H bond activation on the indoles, thus generating various disilylated tetracyclic indolo[2,1-a]isoquinolines in moderate to excellent yields. Furthermore, borylated indolo[2,1-a]isoquinolines can be produced by a Heck/borylation cascade.
中文翻译:

链烯键连接的2-(2-卤代苯基)-1H-吲哚的钯催化的多米诺骨牌Heck-二硅烷化和硼化:获得各种二甲基化和硼化的吲哚[2,1-a]异喹啉
在有机合成中,化学选择性反应仍然是巨大但特别令人着迷的挑战。在本文中,我们报道了在钯催化下烯烃系的2-(2-卤代苯基)-1 H-吲哚与六甲基乙硅烷的新多米诺Heck /二甲硅烷基化反应。该过程涉及一个C–C键和两个C–Si键的形成过程,它通过化学选择性的Heck反应和C–H活化的两个关键步骤有效地进行,通过克服了吲哚上不希望的C3–H键活化,从而生成各种二甲基化的四环吲哚[2,1- a ]异喹啉的产率中等至优异。此外,可以通过Heck /硼酸酯化级联反应产生硼化的吲哚[2,1- a ]异喹啉。
更新日期:2020-07-28
中文翻译:

链烯键连接的2-(2-卤代苯基)-1H-吲哚的钯催化的多米诺骨牌Heck-二硅烷化和硼化:获得各种二甲基化和硼化的吲哚[2,1-a]异喹啉
在有机合成中,化学选择性反应仍然是巨大但特别令人着迷的挑战。在本文中,我们报道了在钯催化下烯烃系的2-(2-卤代苯基)-1 H-吲哚与六甲基乙硅烷的新多米诺Heck /二甲硅烷基化反应。该过程涉及一个C–C键和两个C–Si键的形成过程,它通过化学选择性的Heck反应和C–H活化的两个关键步骤有效地进行,通过克服了吲哚上不希望的C3–H键活化,从而生成各种二甲基化的四环吲哚[2,1- a ]异喹啉的产率中等至优异。此外,可以通过Heck /硼酸酯化级联反应产生硼化的吲哚[2,1- a ]异喹啉。



























 京公网安备 11010802027423号
京公网安备 11010802027423号