当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct catalytic asymmetric and anti-selective vinylogous addition of butenolides to chromones
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0sc01914c Jin Cui 1, 2, 3 , Naoya Kumagai 1, 2, 3 , Takumi Watanabe 1, 2, 3 , Masakatsu Shibasaki 1, 2, 3
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0sc01914c Jin Cui 1, 2, 3 , Naoya Kumagai 1, 2, 3 , Takumi Watanabe 1, 2, 3 , Masakatsu Shibasaki 1, 2, 3
Affiliation
|
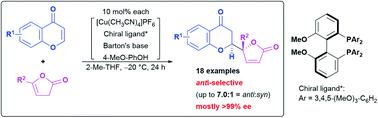
An anti-selective catalytic asymmetric Michael-type vinylogous addition of β,γ-butenolides to chromones was developed. The catalyst system developed herein is characterized by tuning of the steric and electronic effects using a proper Biphep-type chiral ligand to invert the diastereoselection, and improvement of the catalyst turnover by a coordinative phenolic additive. The catalytic protocol renders potentially biologically active natural product analogs accessible in good yield with moderate diastereoselectivity and high enantiomeric purity, mostly greater than 99% ee.
中文翻译:

将丁烯内酯直接催化不对称和反选择性乙烯基键合到色酮上
开发了将β,γ-丁烯内酯向色酮的反选择性催化不对称迈克尔型乙烯基加成物。本文开发的催化剂体系的特征在于使用适当的Biphep型手性配体调节空间和电子效应,以转化非对映异构,并通过配位酚醛添加剂改善催化剂的转化率。催化方案使得潜在的具有生物活性的天然产物类似物可以高收率获得,具有适度的非对映选择性和高对映体纯度,大部分大于99%ee。
更新日期:2020-07-15
中文翻译:

将丁烯内酯直接催化不对称和反选择性乙烯基键合到色酮上
开发了将β,γ-丁烯内酯向色酮的反选择性催化不对称迈克尔型乙烯基加成物。本文开发的催化剂体系的特征在于使用适当的Biphep型手性配体调节空间和电子效应,以转化非对映异构,并通过配位酚醛添加剂改善催化剂的转化率。催化方案使得潜在的具有生物活性的天然产物类似物可以高收率获得,具有适度的非对映选择性和高对映体纯度,大部分大于99%ee。


























 京公网安备 11010802027423号
京公网安备 11010802027423号