当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynamical Trajectory Study of the Transannular [6+4] and Ambimodal Cycloaddition in the Biosynthesis of Heronamides.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.joc.0c01187 Chun Zhang 1 , Xin Wang 1 , Yu Chen 1 , Zhili He 2 , Peiyuan Yu 2 , Yong Liang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.joc.0c01187 Chun Zhang 1 , Xin Wang 1 , Yu Chen 1 , Zhili He 2 , Peiyuan Yu 2 , Yong Liang 1
Affiliation
|
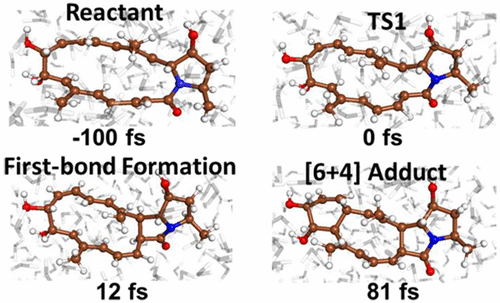
We report the dynamical trajectory study of the transannular [6+4] and ambimodal cycloaddition proposed in the biosynthesis of heronamide natural products. The originally proposed bifurcation of the potential energy surface is found to strongly favor the formation of the [6+4] product, both in the gas phase and in an explicit water environment, as evidenced by our trajectory simulations. The detailed information on how the bonds are formed and how water influences the bonding dynamics was analyzed at the femtosecond time scale.
中文翻译:

跨环[6 + 4]和双峰环加成在Heronamides生物合成中的动力学轨迹研究。
我们报告了跨环[6 + 4]和双峰环加成反应的动力学轨迹研究,该反应在拟合成heronamide天然产物的过程中提出。我们发现,最初提出的势能面的分叉在气相和在明确的水环境中都非常有利于[6 + 4]产物的形成,正如我们的轨迹模拟所证明的那样。在飞秒时间尺度上分析了有关如何形成键以及水如何影响键动力学的详细信息。
更新日期:2020-07-17
中文翻译:

跨环[6 + 4]和双峰环加成在Heronamides生物合成中的动力学轨迹研究。
我们报告了跨环[6 + 4]和双峰环加成反应的动力学轨迹研究,该反应在拟合成heronamide天然产物的过程中提出。我们发现,最初提出的势能面的分叉在气相和在明确的水环境中都非常有利于[6 + 4]产物的形成,正如我们的轨迹模拟所证明的那样。在飞秒时间尺度上分析了有关如何形成键以及水如何影响键动力学的详细信息。


























 京公网安备 11010802027423号
京公网安备 11010802027423号